1-(3-Isoselenocyanatopropyl)adamantane
Abstract
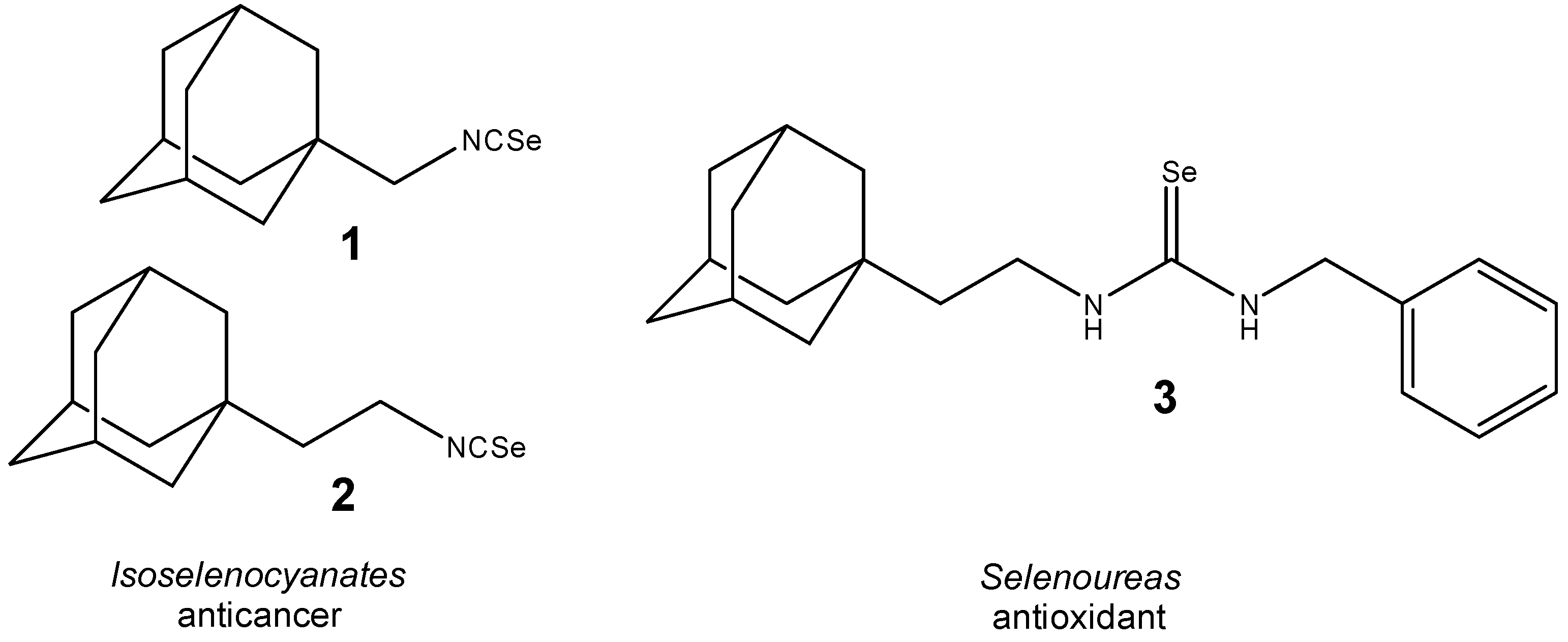
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burmistrov, V.V.; Pitushkin, D.A.; Vasipov, V.V.; D’yachenko, V.S.; Butov, G.M. Synthesis of 3-adamantylated hydantoins and their 2-thio(seleno) analogs. Chem. Heterocycl. Comp. 2019, 55, 619–622. [Google Scholar] [CrossRef]
- Kuznetsov, Y.P.; Rasskazova, E.V.; Pitushkin, D.A.; Eshtukov, A.V.; Vasipov, V.V.; Burmistrov, V.V.; Butov, G.M. Synthesis and Properties of N,N′-Disubstituted Ureas and Their Isosteric Analogs Containing Polycyclic Fragments: XI. 1-[(Adamantan-1-yl)alkyl]-3-arylselenoureas. Russ. J. Org. Chem. 2021, 57, 1036–1046. [Google Scholar] [CrossRef]
- Kuznetsov, Y.P.; Pitushkin, D.A.; Burmistrov, V.V.; Butov, G.M. Synthesis and Properties of N,N′-Disubstituted Ureas and Their Isosteric Analogs Containing Polycyclic Fragments: XV. N,N′-(Alkane-α,ω-diyl)bis[N′-(adamantan-1-yl)]selenoureas. Russ. J. Org. Chem. 2022, 58, 1398–1402. [Google Scholar] [CrossRef]
- Burmistrov, V.; Saxena, R.; Pitushkin, D.; Butov, G.M.; Chung, F.-L.; Aggarwal, M. Adamantyl Isothiocyanates as Mutant p53 Rescuing Agents and Their Structure−Activity Relationships. J. Med. Chem. 2021, 64, 6621–6633. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Saxena, R.; Sinclair, E.; Fu, Y.; Jacobs, A.; Dyba, M.; Wang, X.; Cruz, I.; Berry, D.; Kallakury, B.; et al. Reactivation of mutant p53 by a dietary-related compound phenethyl isothiocyanate inhibits tumor growth. Cell Death Differ. 2016, 23, 1615–1627. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burmistrov, V.; Morisseau, C.; Babkov, D.A.; Golubeva, T.; Pitushkin, D.; Sokolova, E.V.; Vasipov, V.; Kuznetsov, Y.; Bazhenov, S.V.; Novoyatlova, U.S.; et al. Anti-Inflammatory Activity of Soluble Epoxide Hydrolase Inhibitors Based on Selenoureas Bearing an Adamantane Moiety. Int. J. Mol. Sci. 2022, 23, 10710. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Dong, H.; Zhang, X.; Wang, Y.; Su, L.; Xu, H. Selenium as an emerging versatile player in heterocycles and natural products modification. Drug Discov. Today 2022, 27, 2268–2277. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Martin, G.; Plano, D.; Encio, I.; Sanmatrin, C. Novel N,N′–Disubstituted Selenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants 2021, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, J.; Huras, B.; Kiełczewska, A.; Krawczyk, M.; Hupko, J.; Jaszczuk, K. Reactions of Nitroxides, Part 17. Synthesis, Fungistatic and Bacteriostatic Activity of Novel Five- and Six-Membered Nitroxyl Selenoureas and Selenocarbamates. Molecules 2019, 24, 2457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salami, S.A.; Siwe-Noundou, X.; Krause, R.W.M. A More Sustainable Isocyanide Synthesis from N-Substituted Formamides Using Phosphorus Oxychloride in the Presence of Triethylamine as Solvent. Molecules 2022, 27, 6850. [Google Scholar] [CrossRef] [PubMed]
- Biddle, H.C. Ueber Derivate des Isuretins der Formhydroxamsäure und ihre Beziehungen zur Knallsäure. Eur. J. Org. Chem. 1900, 310, 1–24. [Google Scholar] [CrossRef][Green Version]
- Lindemann, H.; Wiegrebe, L. Über die Konstitution der Verbindungen mit “zweiwertigem” Kohlenstoff. Chem. Ber. 1930, 63, 1650–1657. [Google Scholar] [CrossRef]
- Kuznetsov, Y.P.; Pitushkin, D.A.; Eshtukova-Shcheglova, E.A.; Burmistrov, V.V.; Butov, G.M.; Novakov, I.A. Synthesis and antioxidant activity of 1-R-3-(2-fluorophenyl)selenoureas containing polycyclic fragments. Russ. Chem. Bull. 2022, 71, 2467–2472. [Google Scholar] [CrossRef]
- McCullough, C.R.; Pullela, P.K.; Im, S.-C.; Waskell, L.; Sem, D.S. 13C-Methyl isocyanide as an NMR probe for cytochrome P450 active sites. J. Biomol. NMR 2009, 43, 171–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nichugovskiy, A.I.; Khrulev, A.A.; Perevoshchikova, K.A.; Cheshkov, D.A.; Morozova, N.G.; Maslov, M.A. Synthesis of Isonitrile Derivatives of Diglycerides and Carbohydrates as Intermediates for Multicomponent Ugi Reaction. Russ. J. Bioorg. Chem. 2021, 47, 929–938. [Google Scholar] [CrossRef]
- Ivanenkov, Y.A.; Veselov, M.S.; Rezekin, I.G.; Skvortsov, D.A.; Sandulenko, Y.B.; Polyakova, M.V.; Bezrukov, D.S.; Vasilevsky, S.V.; Kukushkin, M.E.; Moiseeva, A.A.; et al. Synthesis, isomerization and biological activity of novel 2-selenohydantoin derivatives. Bioorg. Med. Chem. 2016, 24, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hiraoka, T.; Ishiguro, F.; Jeona, J.-H.; Chujo, Y. Adamantane ionic liquids. RSC Adv. 2014, 4, 28107–28110. [Google Scholar] [CrossRef][Green Version]
- Novakov, I.A.; Kulev, I.A.; Radchenko, S.S.; Birzniens, K.A.; Boreiko, E.I.; Vladyko, G.V.; Korobchenko, L.V. Synthesis and antiviral activity of the hydrochlorides of alicyclic mono- and diamines. Pharm Chem. J. 1987, 21, 287–291. [Google Scholar] [CrossRef]



| Alcohol | Time, h | The Content of Compound 5 in the Reaction Mass, % |
|---|---|---|
| methanol | 8 | 23 |
| ethanol | 8 | 49 |
| i-propanol | 8 | 65 |
| tert-butanol | 8 | 88 |
| 1-octanol | 8 | 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitushkin, D.; Burmistrov, V.; Butov, G. 1-(3-Isoselenocyanatopropyl)adamantane. Molbank 2023, 2023, M1646. https://doi.org/10.3390/M1646
Pitushkin D, Burmistrov V, Butov G. 1-(3-Isoselenocyanatopropyl)adamantane. Molbank. 2023; 2023(2):M1646. https://doi.org/10.3390/M1646
Chicago/Turabian StylePitushkin, Dmitry, Vladimir Burmistrov, and Gennady Butov. 2023. "1-(3-Isoselenocyanatopropyl)adamantane" Molbank 2023, no. 2: M1646. https://doi.org/10.3390/M1646
APA StylePitushkin, D., Burmistrov, V., & Butov, G. (2023). 1-(3-Isoselenocyanatopropyl)adamantane. Molbank, 2023(2), M1646. https://doi.org/10.3390/M1646







