Synthesis and Structure of N-(1-(Bromomethyl)-7,7-dimethylbicyclo[2.2.1]heptan-2-yl)benzenesulfonamide
Abstract
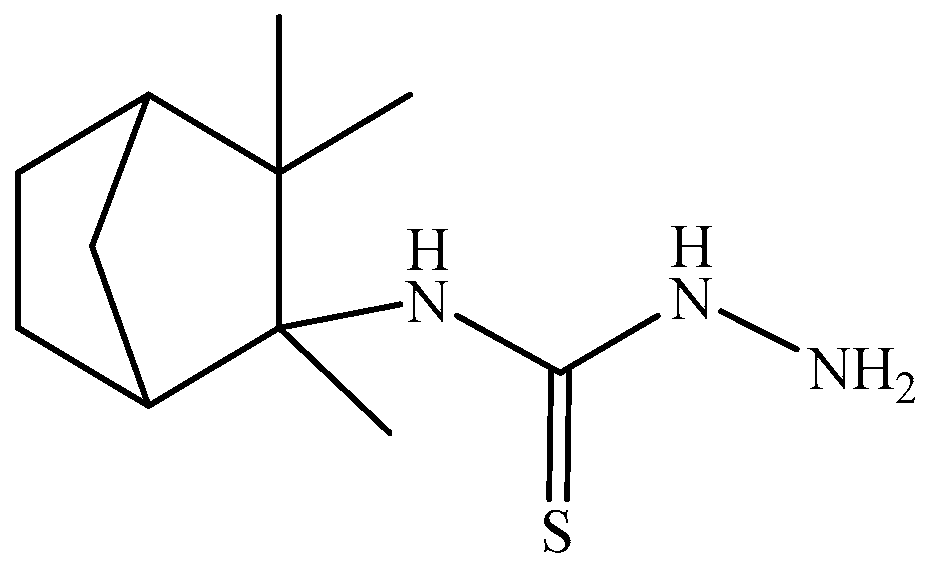
1. Introduction
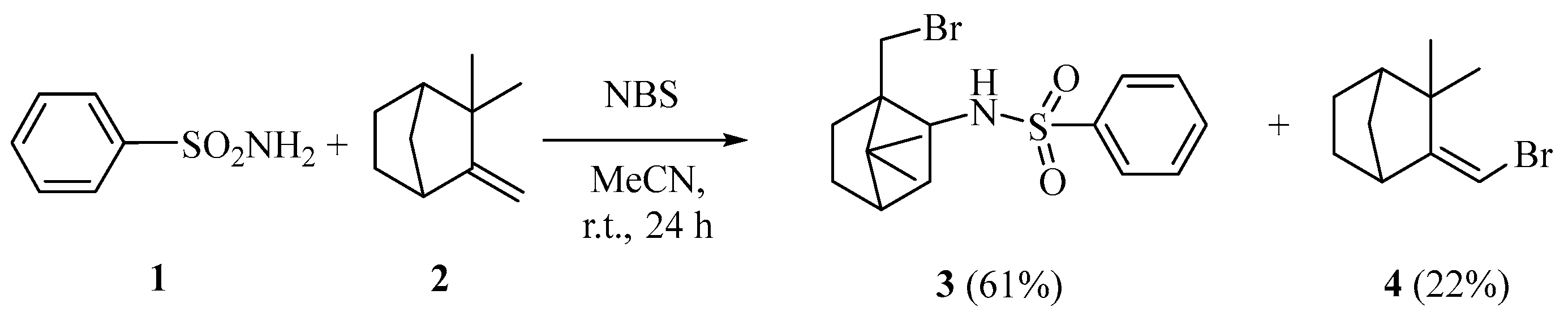
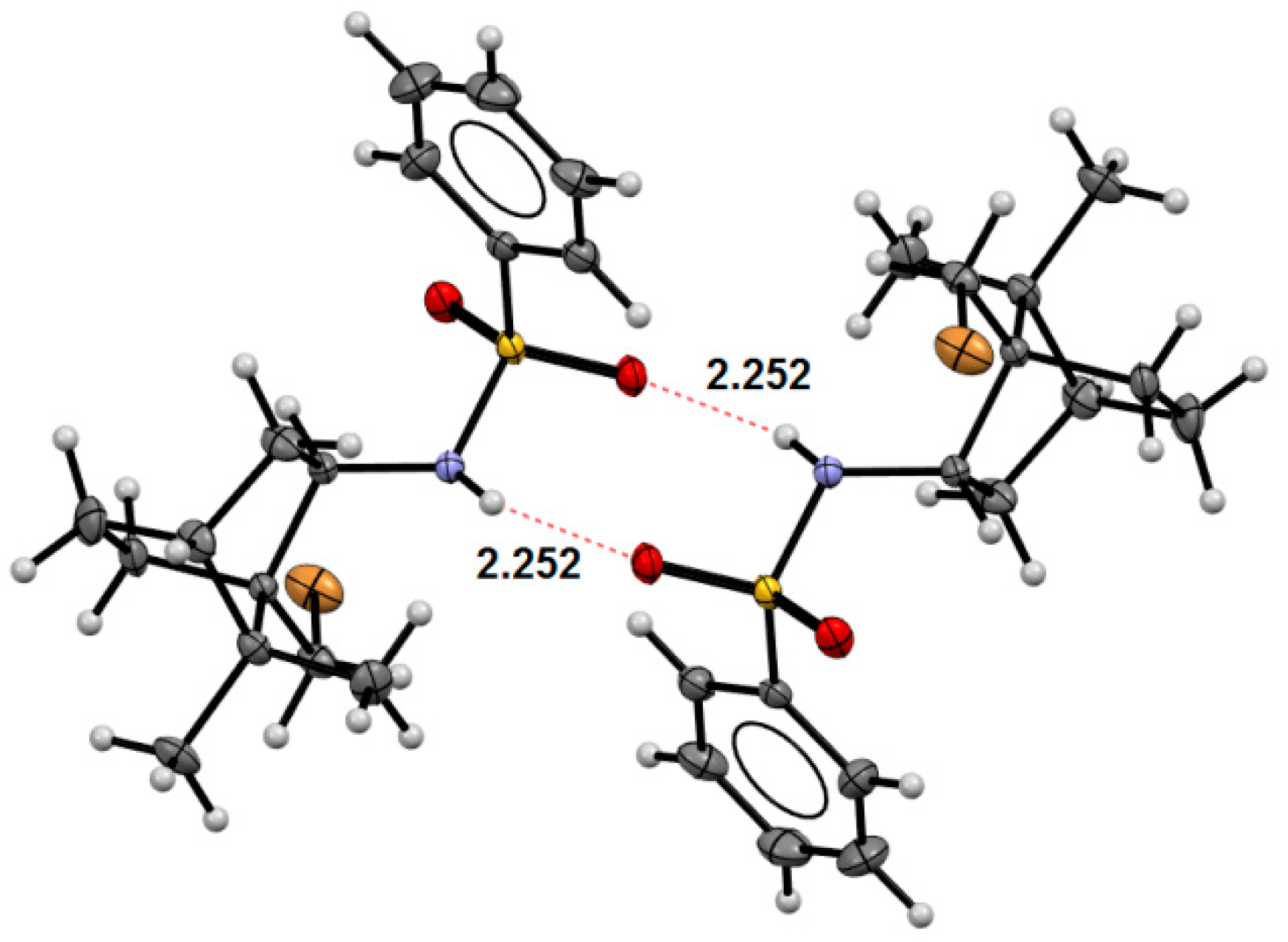
2. Results and Discussion
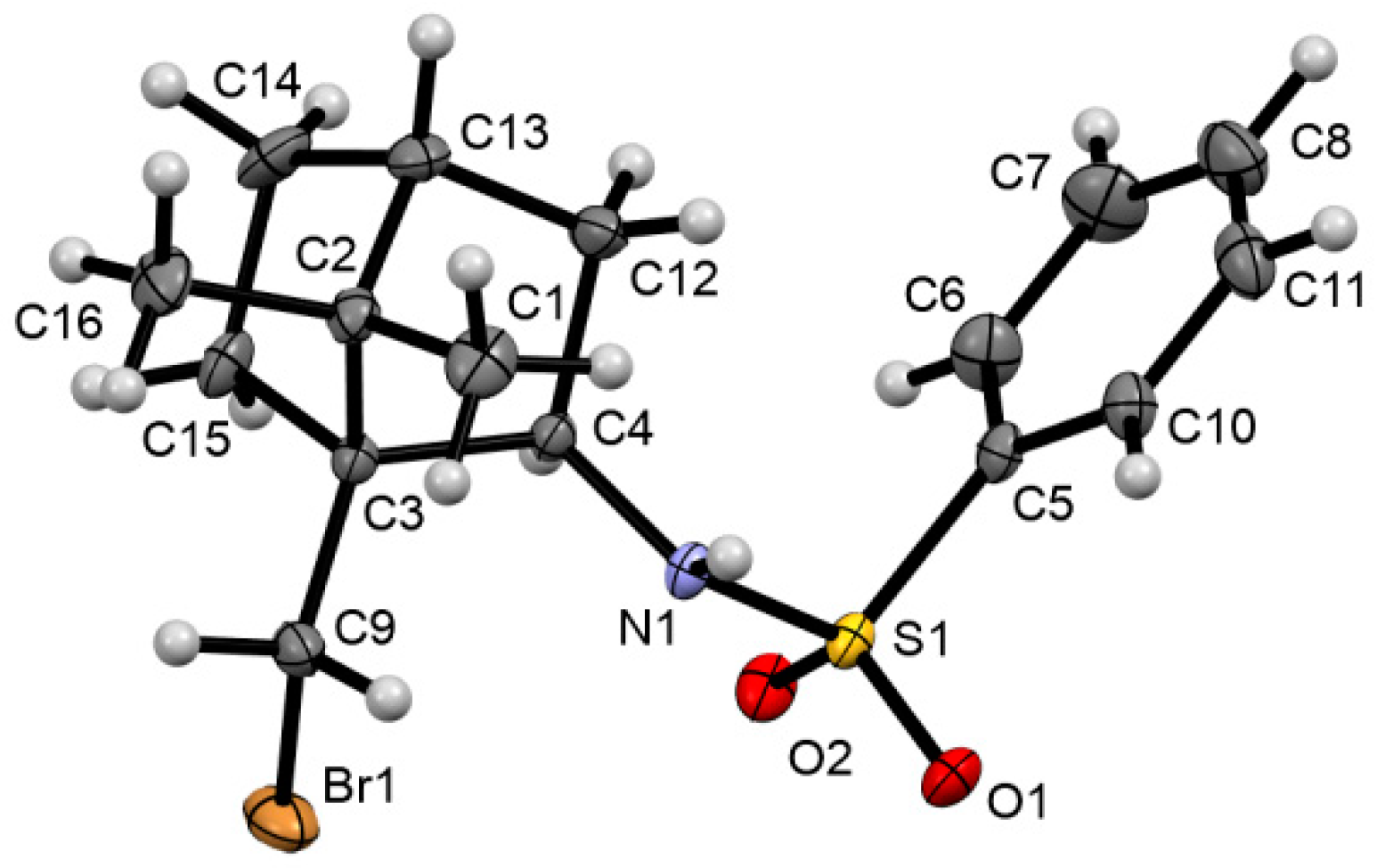
Crystal Structure
3. Materials and Methods
3.1. General Information
3.2. Synthesis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baek, S.; Kim, J.; Moon, B.S.; Park, S.M.; Jung, D.E.; Kang, S.Y.; Lee, S.J.; Oh, S.J.; Kwon, S.H.; Nam, M.H.; et al. Camphene Attenuates Skeletal Muscle Atrophy by Regulating Oxidative Stress and Lipid Metabolism in Rats. Nutrients 2020, 12, 3731. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, B.C.; Queiroz, P.A.; Baldin, V.P.; do Amaral, P.H.R.; Rodrigues, L.L.F.; Vandresen, F.; R Caleffi-Ferracioli, K.; de L Scodro, R.B.; Cardoso, R.F.; Siqueira, V.L.D. (-)-Camphene-based derivatives as potential antibacterial agents against Staphylococcus aureus and Enterococcus spp. Future Microbiol. 2020, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.; Moreira, J.C.F.; Pasquali, M.A.B.; Rabie, S.M.S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.A.; Lima, P.S.S.; Cavalcanti, S.C.H.; et al. Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in Experimental Models. ISRN Toxicol. 2013, 2013, 459530. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, H.; Xia, D.; Wang, S. Antioxidant Properties of Camphene-Based Thiosemicarbazones: Experimental and Theoretical Evaluation. Molecules 2020, 25, 1192. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Putilova, V.P.; Yarovaya, O.I.; Zybkina, A.V.; Mordvinova, E.D.; Zaykovskaya, A.V.; Shcherbakov, D.N.; Orshanskaya, I.R.; Sinegubova, E.O.; Esaulkova, I.L.; et al. Synthesis and Antiviral Activity of Camphene Derivatives against Different Types of Viruses. Molecules 2021, 26, 2235. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Govindarajan, M.; Rajeswary, M.; Vaseeharan, B.; Alyahya, S.A.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Maggi, F. Insecticidal activity of camphene, zerumbone and α-humulene from Cheilocostus speciosus rhizome essential oil against the Old-World bollworm, Helicoverpa armigera. Ecotoxicol. Environ. Saf. 2018, 148, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.U.; Barbosa da Silva, A.P.; Ueda-Nakamura, T.; Dias Filho, B.P.; Conceição da Silva, C.; Nakamura, C.V. Effects of a thiosemicarbazide camphene derivative on Trichophyton mentagrophytes. Molecules 2009, 14, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.J.; de Andrade Leles, L.C.; Ferreira, S.O.; da Silva, R.C.; de V. Viveiros, K.; Chaves, D.M.; Pinheiro, P.F. A Rare Carbon Skeletal Oxidative Rearrangement of Camphene Catalyzed by Al-Exchanged Keggin Heteropolyacid Salts. ChemistrySelect 2019, 4, 7665–7672. [Google Scholar] [CrossRef]
- Dağalan, Z.; Koçak, R.; Daştan, A.; Nişancı, B. Selectfluor and TBAX (Cl, Br) Mediated Oxidative Chlorination and Bromination of Olefins. Org. Lett. 2022, 24, 8261–8264. [Google Scholar] [CrossRef] [PubMed]
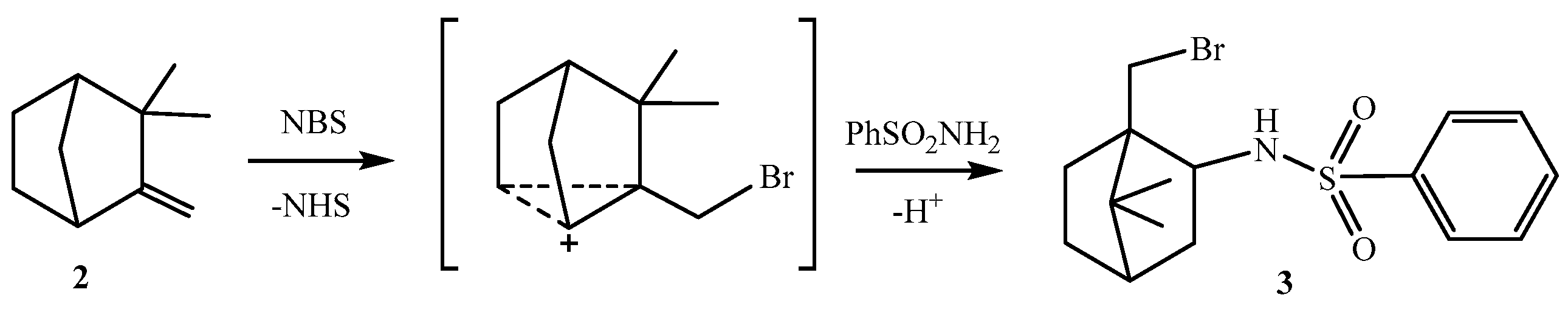
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garagan, I.A.; Moskalik, M.Y.; Sterkhova, I.V.; Ganin, A.S. Synthesis and Structure of N-(1-(Bromomethyl)-7,7-dimethylbicyclo[2.2.1]heptan-2-yl)benzenesulfonamide. Molbank 2023, 2023, M1645. https://doi.org/10.3390/M1645
Garagan IA, Moskalik MY, Sterkhova IV, Ganin AS. Synthesis and Structure of N-(1-(Bromomethyl)-7,7-dimethylbicyclo[2.2.1]heptan-2-yl)benzenesulfonamide. Molbank. 2023; 2023(2):M1645. https://doi.org/10.3390/M1645
Chicago/Turabian StyleGaragan, Ivan A., Mikhail Yu. Moskalik, Irina V. Sterkhova, and Anton S. Ganin. 2023. "Synthesis and Structure of N-(1-(Bromomethyl)-7,7-dimethylbicyclo[2.2.1]heptan-2-yl)benzenesulfonamide" Molbank 2023, no. 2: M1645. https://doi.org/10.3390/M1645
APA StyleGaragan, I. A., Moskalik, M. Y., Sterkhova, I. V., & Ganin, A. S. (2023). Synthesis and Structure of N-(1-(Bromomethyl)-7,7-dimethylbicyclo[2.2.1]heptan-2-yl)benzenesulfonamide. Molbank, 2023(2), M1645. https://doi.org/10.3390/M1645






