Abstract
Multicomponent reactions have been demonstrated as a promising tool for the creation of diverse molecular structures with enhanced efficiency, reduced waste, and a high atom economy. Arylglyoxal monohydrates with two different carbonyl groups are well known as worthwhile synthons in organic synthesis. 2-Pyrone and pyrimidine-2,4,6-trione are versatile building blocks for the synthesis of key intermediates in synthetic organic chemistry as well as in medicinal chemistry. A simple and efficient tandem Knoevenagel–Michael protocol for the synthesis of the previously unknown 5-(1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimet-hylpyrimidine-2,4,6(1H,3H,5H)-trione was elaborated. The suggested method is based on the multicomponent reaction of phenylglyoxal hydrate, 1,3-dimethylbarbituric acid, and 4-hydroxy-6-methyl-2H-pyran-2-one. The structure of the synthesized compound was proven by 1H, 13C-NMR, and IR spectroscopy, mass spectrometry, and elemental analysis. A procedure for predicting the possible types of its biological activity was carried out for the title compound.
1. Introduction
Multicomponent reactions (MCRs) are reactions that involve the formation of multiple bonds in a single operation. MCRs have been demonstrated as a promising tool for the creation of diverse molecular structures with enhanced efficiency, reduced waste, and high atom economy from easily accessible and inexpensive starting materials by simple mixing of the reactant [1,2]. The ability to obtain the desired compound in “one-pot” with an operationally simple workup without the use of complex purification methods and the avoidance of the isolation of the reaction intermediate is a powerful strategy for green or sustainable synthesis [3].
Arylglyoxal monohydrates with two different carbonyl groups are well known as worthwhile synthons in organic synthesis, especially in the one-pot and multicomponent preparation of heterocyclic frameworks [4]. The presence of an electron-withdrawing ketone group adjacent to the aldehyde caused greater reactivity of the aldehyde group than benzaldehyde. In addition, the presence of the above-mentioned carbonyl groups in the chemical structure of arylglyoxal monohydrates causes superior reactivity and selectivity during the reaction process [5].
2-Pyrone is a versatile building block for the synthesis of key intermediates in synthetic organic chemistry as well as in medicinal chemistry due to the existence of functional groups such as conjugated diene and the ester group [6]. Thus, the development of a highly efficient synthetic method affording substituted 2-pyrones under mild conditions has attracted considerable attention in organic chemistry [7].
Pyrimidine-2,4,6-trione has been utilized in the design and synthesis of diverse types of compounds and is considered an important building block in organic synthesis [8]. Barbiturates have a special place in pharmaceutical chemistry because of their biological activities such as sedative [9], anticonvulsant [10], antimicrobial [11], and anticancer [12] properties.
Therefore, the elaboration of novel synthetic method based on the multicomponent reaction of arylglyoxals, 2-pyrones, and pyrimidine-2,4,6-triones is of great interest.
2. Results and Discussion
Herein, we develop an efficient tandem Knoevenagel–Michael approach to synthesize 5-(1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethyl-pyrimidine-2,4,6(1H,3H,5H)-trione (4) on the basis of the multicomponent reaction of phenylglyoxal hydrate (1), 1,3-dimethylbarbituric acid (2), and 4-hydroxy-6-methyl-2H-pyran-2-one (3) (Scheme 1).

Scheme 1.
Multicomponent reaction of phenylglyoxal hydrate (1), 1,3-dimethylbarbituric acid (2), and 4-hydroxy-6-methyl-2H-pyran-2-one (3).
We have previously demonstrated that the syntheses of such symmetrical and unsymmetrical compounds can be achieved using various techniques and reaction systems [13,14,15,16,17,18]. In addition, we recently carried out a multicomponent transformation of benzaldehydes, 1,3-dimethylbarbituric acid 2, and 4-hydroxy-6-methyl-2H-pyran-2-one (3) by reflux in ethanol for 2 h in the presence of 10 mol% sodium acetate [19].
In this research, the transformation of phenylglyoxal hydrate (1), 1,3-dimethylbarbituric acid (2), and 4-hydroxy-6-methyl-2H-pyran-2-one (3) in the presence of p-toluenesulfonic acid (pTSA) monohydrate in ethanol for 4 h under reflux resulted in 5-(1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4).
When the reaction was finished, the reaction mixture was evaporated to dryness in a rotary evaporator vacuum. A small amount of chloroform was added to the resulting residue and stirred for 15 min at ambient temperature. The resulting precipitate of ((2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyrimidine 4 in pure form was filtered off and dried in the vacuum of a water jet pump. Final compound 4 was obtained at an 83% yield.
When we carried out the reaction under the conditions described in the article [19], the target compound 4 was obtained with a yield of 75%.
The bond-forming index (BFI) of this process was two, because two new bonds were formed in one stage, namely, two C-C bonds.
The structure of novel ((2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyrimi-dine 4 was established using 1H, 13C NMR, and IR spectroscopy, mass spectrometry data, and elemental analysis (see Supplementary Materials).
Taking into consideration the known data on tandem Knoevenagel–Michael reactions [13,19,20], a multicomponent transformation mechanism was proposed (Scheme 2). The process begins with the formation of phenylglyoxal (5) from its hydrate 1. Under these conditions, the condensation of phenylglyoxal (5) and 1,3-dimethylbarbituric acid (2) results in the formation of the Knoevenagel adduct 6. The following Michael addition of 4-hydroxy-6-methyl-2H-pyran-2-one 3 affords the formation of 5-(1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4).
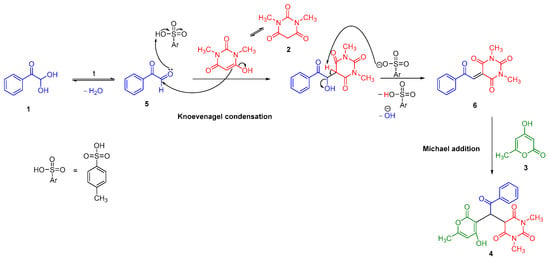
Scheme 2.
A plausible mechanism for the formation of ((2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyrimidine 4.
We did not detect any intermediates during the reaction, so the mechanism was proposed based on the pKa values of C-H acids 2 and 3. 1,3-Dimethylbarbituric acid (2) (pKa 4.68 [21]) has a lower pKa value than 4-hydroxy-6-methyl-2H-pyran-2-one (3) (pKa 6.83 [22]), so it is a stronger acid and reacts with the carbonyl compound 1 first.
Because the target compound 4 contains two pharmacophore fragments, we carried out a procedure for predicting the possible types of its biological activity. To do this, we used the drug design software PASS (Prediction of Activity Spectra for Substances) [23,24]. Calculations showed that ((2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyri-midine 4 is promising for further research as an anaphylatoxin receptor antagonist, protein CYP2H substrate, platelet aggregation inhibitor, and kidney function stimulant.
3. Materials and Methods
3.1. General Methods
All reagents and solvents were purchased from commercial sources without further purification.
The melting point was taken in open capillary tubes on the Gallenkamp melting-point apparatus (Gallenkamp & Co., Ltd., London, UK). 1H and 13C NMR spectra were recorded with a Bruker AM300 spectrometer (Bruker Corporation, Billerica, MA, USA) at ambient temperature in DMSO-d6 solution. The correlation of signals in the description of the spectra is not unambiguous. The IR spectrum (KBr pellets) was obtained on a Bruker ALPHA-T FT-IR spectrometer (Bruker Corporation, Billerica, MA, USA). The mass spectrum (EI = 70 eV) was registered on a Kratos MS-30 spectrometer (Kratos Analytical Ltd., Manchester, UK). Elemental analyzer 2400 (Perkin Elmer Inc., Waltham, MA, USA) was applied for elemental analysis.
3.2. Synthesis of 5-(1-(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4)
Phenylglyoxal hydrate (1) (0.152 g, 1 mmol), 1,3-dimethylbarbituric acid (2) (0.156 g, 1 mmol), 4-hydroxy-6-methyl-2H-pyran-2-one (3) (0.126 g, 1 mmol), and p-toluenesulfonic acid monohydrate (0.019 g, 0.1 mmol) were refluxed in 5 mL of EtOH for 4 h. After the reaction was finished, the reaction mixture was evaporated to dryness on a rotary evaporator. An amount of 3 mL of chloroform was added to the residue, and this mass was stirred for 15 min at ambient temperature. The precipitate was filtered, washed with chloroform (2 mL × 2), and dried to isolate pure 5-(1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4).
5-(1-(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4). White crystals; yield 83% (0.330 g); mp = 161–163 °C (from CHCl3); FTIR (KBr) cm−1: 3032 (C-H arom.), 1749 (C=O pyrone), 1677 (C=O b. acid), 1574 (C=C Ar), 1448 (C=C Ar), 1374 (CH3), 1255 (C-O), 756 (C-H arom.). 1H NMR (300 MHz, DMSO-d6): δ 2.18 (s, 3H, CH3 pyr), 3.15 (s, 6H, 2 N-CH3), 3.98 (d, 1H, 3J = 3.9 Hz, CH b.acid), 5.35 (d, 1H, 3J = 3.9 Hz, C(2)H), 6.00 (s, 1H, CH pyr), 7.46 (t, 2H, 3J = 7.4 Hz, 2 CH Ph), 7.56 (t, 1H, 3J = 7.4 Hz, CH Ph), 7.72 (d, 2H, 3J = 7.4 Hz, 2 CH Ph), 11.92 (s, 1H, OH) ppm; 13C NMR (75 MHz, DMSO-d6): δ 19.2 (CH3 pyr), 27.9 (N-CH3), 28.1 (N-CH3), 46.7 (C(2)H), 47.2 (CH b.acid), 98.7 (CH pyr), 99.7 (C(3) pyr), 127.1 (2C, 2 o-CH Ph), 128.6 (2C, 2 m-CH Ph), 132.9 (p-CH Ph), 136.0 (C Ph), 151.8 (C(2)=O b.acid), 161.8 (C=O pyr), 163.1 (C(6)-CH3 pyr), 165.7 (C(4)-OH pyr), 166.9 (C(4)=O b.acid), 168.4 (C(6)=O b.acid), 197.2 (C(2)=O gl.) ppm; MS (m/z, relative intensity %): 398 [M]+ (100), 271 [M–H2O]+ (87), 293 [M–C7H5O]+ (5), 272 [M–C6H6O3]+ (1), 105 [C7H5O]+ (20), 77 [C6H5]+ (15); Anal. calcd. for C20H18N2O7: C, 60.30; H, 4.55; N, 7.03%; found: C, 60.34; H, 4.59; N, 6.94%.
4. Conclusions
In summary, a simple and efficient multicomponent protocol for the synthesis of an earlier unknown 5-(1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4) on the basis of the interaction of phenylglyoxal hydrate (1), 1,3-dimethylbarbituric acid (2), and 4-hydroxy-6-methyl-2H-pyran-2-one (3) was reported. The presented method may be utilized to synthesize a wide variety of similar substances due to the use of readily available starting materials, atom economy, and a feasible work-up process. The structure of the obtained compound was established by 1H and 13C NMR and IR spectroscopy, mass spectrometry, and elemental analysis.
A procedure for predicting the possible types of its biological activity was carried out for the title compound. ((2H-Pyran-3-yl)-2-oxo-2-phenylethyl)-1,3-dimethylpyrimi-dine 4 is promising for further research as an anaphylatoxin receptor antagonist, protein CYP2H substrate, platelet aggregation inhibitor, and kidney function stimulant.
Supplementary Materials
The following are available online. Compound 4 spectra: 1H NMR (Figure S1), 13C NMR (Figure S2), IR (Figure S3), MS (EI) (Figure S4).
Author Contributions
Conceptualization, Y.E.R. and M.N.E.; methodology, Y.E.R.; validation, Y.E.R., F.V.R. and M.N.E.; formal analysis, V.M.K.; investigation, V.M.K.; data curation, F.V.R. and M.N.E.; writing—original draft preparation, Y.E.R.; writing—review and editing, F.V.R. and M.N.E.; supervision, M.N.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data for the compound presented in this study are available in the Supplementary Materials of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Toure, B.B.; Hall, D.G. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 2009, 109, 4439–4486. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, D.; Castillo, J.; Becerra, D.; Rojas, H.; Abonia, R. Synthesis of biologically active molecules through multicomponent reactions. Molecules 2020, 25, 505. [Google Scholar] [CrossRef] [PubMed]
- de Graaff, C.; Ruijter, E.; Orru, R.V. Recent developments in asymmetric multicomponent reactions. Chem. Soc. Rev. 2012, 41, 3969–4009. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari-Sis, B.; Zirak, M.; Akbari, A. Arylglyoxals in synthesis of heterocyclic compounds. Chem. Rev. 2013, 113, 2958–3043. [Google Scholar] [CrossRef]
- Mousavi, H. A concise and focused overview upon arylglyoxal monohydrates-based one-pot multi-component synthesis of fascinating potentially biologically active pyridazines. J. Mol. Struct. 2022, 1251, 131742. [Google Scholar] [CrossRef]
- Lee, J.S. Recent advances in the synthesis of 2-pyrones. Mar. Drugs 2015, 13, 1581–1620. [Google Scholar] [CrossRef]
- Dobler, D.; Leitner, M.; Moor, N.; Reiser, O. 2-Pyrone—A Privileged Heterocycle and Widespread Motif in Nature. Eur. J. Org. Chem. 2021, 2021, 6180–6205. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Aleali, F.; Lashgari, N. Recent applications of barbituric acid in multicomponent reactions. RSC Adv. 2016, 6, 50895–50922. [Google Scholar] [CrossRef]
- Kliethermes, C.L.; Metten, P.; Belknap, J.K.; Buck, K.J.; Crabbe, J.C. Selection for pentobarbital withdrawal severity: Correlated differences in withdrawal from other sedative drugs. Brain Res. 2004, 1009, 17–25. [Google Scholar] [CrossRef]
- Archana; Srivastava, V.K.; Kumar, A. Synthesis of some newer derivatives of substituted quinazolinonyl-2-oxo/thiobarbituric acid as potent anticonvulsant agents. Bioorg. Med. Chem. 2004, 12, 1257–1264. [Google Scholar] [CrossRef]
- Dhorajiya, B.D.; Dholakiya, B.Z.; Mohareb, R.M. Hybrid probes of aromatic amine and barbituric acid: Highly promising leads for anti-bacterial, anti-fungal and anti-cancer activities. Med. Chem. Res. 2014, 23, 3941–3952. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, M.; Verma, P. Design, synthesis and anticancer activities of hybrids of indole and barbituric acids--identification of highly promising leads. Bioorg. Med. Chem. Lett. 2009, 19, 3054–3058. [Google Scholar] [CrossRef] [PubMed]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Leonova, N.A.; Egorov, M.P. On water noncatalytic tandem Knoevenagel–Michael reaction of aldehydes, N,N′-dimethylbarbituric acid and cyclohexane-1,3-diones. Mendeleev Commun. 2020, 30, 15–17. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Ryzhkova, Y.E.; Krymov, S.K.; Leonova, N.A.; Goloveshkin, A.S.; Egorov, M.P. Electrocatalytic one-pot multicomponent assembly of aldehydes, 2,4-dihydro-3H-pyrazol-3-ones and kojic acid. Mendeleev Commun. 2020, 30, 223–225. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkova, Y.E.; Krymov, S.K.; Vereshchagin, A.N.; Goloveshkin, A.S.; Egorov, M.P. Electrochemically induced multicomponent ‘one-pot’ assembling benzaldehydes, N,N′-dimethylbarbituric acid, and kojic acid. Monatsh. Chem. 2020, 151, 567–573. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkova, Y.E.; Ryzhkov, F.V.; Vereshchagin, A.N.; Leonova, N.A.; Egorov, M.P. Electrochemically Induced Facile and Efficient Multicomponent Approach to Medicinally Relevant 4-[4-oxo-4H-pyran-2-yl](aryl)-methylisoxazol-5(2H)-one Scaffold. ChemistrySelect 2020, 5, 5981–5986. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkova, Y.E.; Vereshchagin, A.N.; Leonova, N.A.; Minaeva, A.P.; Egorov, M.P. Electrochemically induced tandem Knoevenagel-Michael assembling of aldehydes with kojic acid: Direct and efficient arylbis[3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl]methanes formation. Arkivoc 2020, 7, 201–213. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Ryzhkova, Y.E.; Karpenko, K.A.; Ryzhkov, F.V.; Egorov, M.P. Electrocatalytic tandem assembly of aldehydes with 2-thiobarbituric acid into 5,5′-(arylmethylene)bis(1,3-diethyl-2-thiobarbituric acids) and evaluation of their interaction with catalases. Chem. Heterocycl. Comp. 2021, 57, 274–283. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Kalashnikova, V.M.; Fakhrutdinov, A.N.; Elinson, M.N. Green multicomponent approach to novel 5-[(2H-Pyran-3-yl)(aryl)methyl]-1,3-dimethylpyrimidines. J. Heterocycl. Chem. 2022, 1, 576–584. [Google Scholar] [CrossRef]
- Elinson, M.N.; Dorofeev, A.S.; Nasybullin, R.F.; Nikishin, G.I. Facile and Convenient Synthesis of 4,4′-(Arylmethylene)bis(1H-pyrazol-5-ols) by Electrocatalytic Tandem Knoevenagel-Michael Reaction. Synthesis 2008, 12, 1933–1937. [Google Scholar] [CrossRef]
- Korotkikh, N.I.; Cowley, A.H.; Moore, J.A.; Glinyanaya, N.V.; Panov, I.S.; Rayenko, G.F.; Pekhtereva, T.M.; Shvaika, O.P. Reaction of 1-tert-butyl-3,4-diphenyl-1,2,4-triazol-5-ylidenes with a malonic ester. Org. Biomol. Chem. 2008, 6, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-F.; Ang, K.-P.; Jayachandran, H. Ionization constants of some hydroxypyrones in water and in 80% (w/w) dimethyl sulphoxide–water at 25 °C. J. Chem. Soc. Perkin Trans. 2 1983, 4, 471–473. [Google Scholar] [CrossRef]
- Web Site for PASS. Available online: http://way2drug.com/passonline/predict.php (accessed on 17 March 2023).
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. Prediction of biological activity spectra for substances: Evaluation on the diverse set of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).