Abstract
In this paper, we report (η5-pentamethylcyclopentadienyl)iridium{1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazolin-2-ylidene}dichloride, 2, synthesized using a silver transfer agent generated from 1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazolium bromide. Compound 2 was characterized using 1H and 19F NMR spectroscopy and mass spectrometry, and its structure was determined in a single-crystal X-ray diffraction study.
1. Introduction
Group 9 metal complexes of N-heterocycle carbene (NHC) ligands bearing polyfluoroaryl substituents have provided a fertile area of research recently. Importantly, these complexes possess catalytic properties [1,2], including for base-free dynamic resolution in combination with an enzyme [3], and display unusual reactivity [4,5,6,7], including carbon–fluorine bond activation [8,9]. The structures of some of the complexes have been determined in single-crystal X-ray diffraction studies [5,6,7,8,9,10,11,12,13], but of these, most complexes contain a monofluorinated substituent, and only four bear a perfluorinated aryl substituent. The crystal structure of the dichloromethane solvate of the complex Cp*IrCl2{κC-C6H5CH2NC3H2NCH2C6F5} (1) (Figure 1) [13] display a number of short intermolecular distances that suggest significant attractive interactions: π–π stacking between the phenyl ring of molecule of 1 and the pentafluorophenyl ring of another, hydrogen bonding between the hydrogen atoms of the imidazolin-2-ylidene ring of one molecule of 1 and the chloride ligands of another, hydrogen bonding between the hydrogen atoms of a dichloromethane molecule and the chloride ligands, and an interaction between one of the chlorine atoms of dichloromethane and the C5 ring of the pentamthylcyclopentadienyl ligand of 1. Here, we report the analogous iridium complex of the NHC ligand bearing two pentafluorobenzyl substituents (2) (Figure 1).
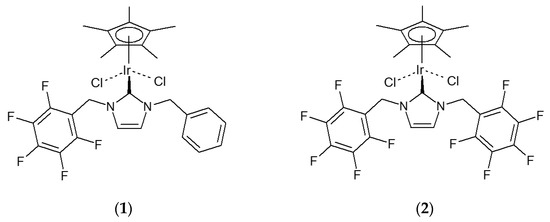
Figure 1.
Compounds 1 and 2.
2. Results
The title compound (2) was synthesized in two steps from 1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazolium bromide [14]. Treatment of this salt with silver oxide afforded the silver carbene complex [AgBr{κC-1,3-(C6F5CH2)2N2C3H2}], which was added to (η5-pentamethylcyclopentadienyl)iridium dichloride dimer (Scheme 1). As expected, the 1H NMR spectrum is simple, displaying just four resonances: two singlets arising from the methyl (δ 1.76) and imidazolin-2-ylidene ring hydrogen atoms (δ 6.52), and two mutually coupled doublets assigned to the diastereotopic methylene hydrogen atoms (δ 6.20 and 5.51). The 19F NMR spectrum is also simple, possessing three resonances assigned to the ortho (δ −140.39), meta (δ −159.64) and para (δ −150.91) fluorine atoms. The mass spectrum shows the cation on loss of chloride (see Supplementary Materials).
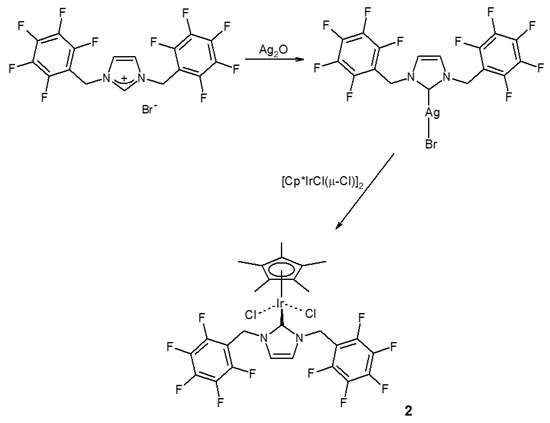
Scheme 1.
Synthesis of (η5-pentamethylcyclopentadienyl)iridium{1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazoline-2-ylidene}dichloride (2).
Compound 2 crystallized as a monosolvate from chloroform in the orthorhombic space group Pnam. The molecules of both 2 and chloroform lie on the crystallographic mirror plane (Figure 2). Selected distances and angles are given in Table 1. The Cp*—Ir, Ir—Cl and Ir—C(NHC) distances and Cp*—Ir—Cl, Cp*—Ir—C and Cl—Ir—C(NHC) angles are consistent with those of 1.
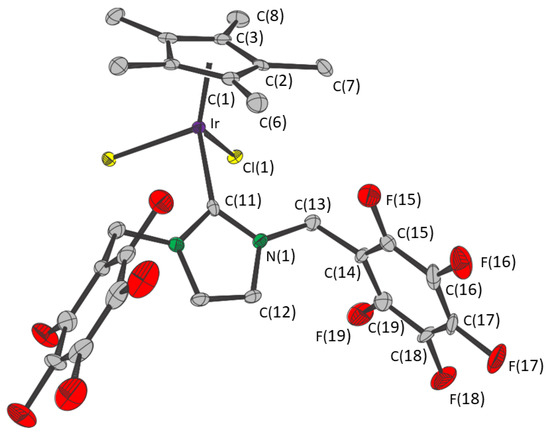
Figure 2.
Structure of compound 2. Thermal ellipsoids are at the 50% level. Hydrogen atoms and the chloroform molecule are omitted for clarity. The two halves of the molecule are related by a mirror plane through Ir, C(1), C(6) and C(11).

Table 1.
Selected distances (Å) and angles (°) of 2.CHCl3.
The crystal structure comprises columns of alternating molecules of 2 and chloroform parallel to the b axis. There are short distances between the molecules of 2 and adjacent chloroform molecules evident in the crystal structure (Figure 3). One molecule of chloroform is positioned close to the pentamethyl cyclopentadienyl ring with the plane defined by the three chlorine atoms almost parallel (ca. 1.7°) to that of the C5 ring. The Cl(1S)···Cp*, Cl(2S)···C(6) and Cl(2S)···C(6) distances (Table 1) are 0.1, 0.3 and 0.4 Å greater than the sum of the van der Waals radii of carbon and chlorine (3.45 Å) [15]. The other molecule of chloroform is positioned close to the chloride ligands. The C(1S)···Cl(1) distance suggests bifurcated hydrogen bonding between the molecules of chloroform and 2. A similar interaction involving one of the hydrogen atoms of the dichloromethane molecule is present in the crystal structure of 1.CH2Cl2.
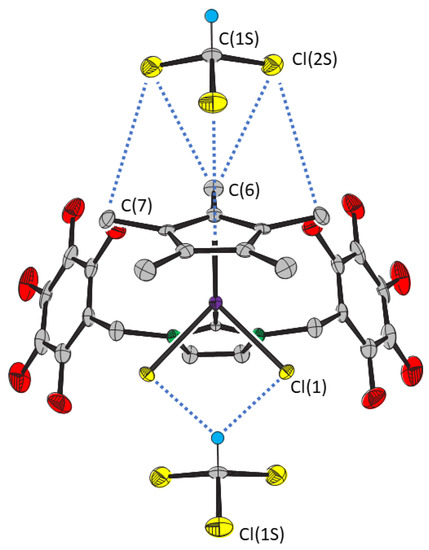
Figure 3.
Close contacts between compound 2 and the chloroform molecules. Thermal ellipsoids are at the 50% level. Hydrogen atoms, except those involved in intermolecular interactions, are omitted for clarity.
The columns are linked parallel to the a axis by short distances between the chloride and imidazolin-2-ylidene ligands of molecules of 2 (Figure 4). The Cl(1)···C(12) distances are the same as the sum of the van der Waals radii of carbon and chlorine [15], which, together with the associated angles, strongly suggest two hydrogen bonds between the two molecules of 2. A similar interaction is evident in the crystal structure of 1.CH2Cl2. Thus, the chloride ligands of 1 and 2 are hydrogen bond acceptors to two species with hydrogen bonds that are mutually perpendicular and also perpendicular to the Ir—Cl(1) bond.
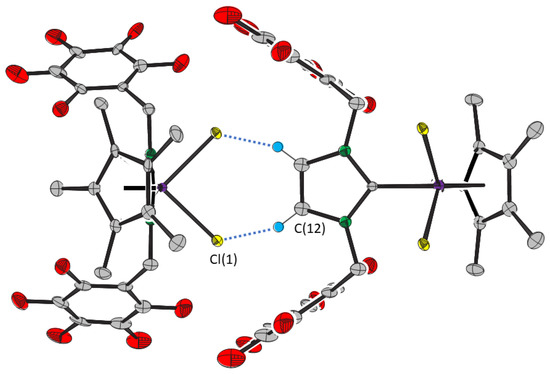
Figure 4.
Close contacts between molecules of compound 2. Thermal ellipsoids are at the 50% level. Hydrogen atoms, except those involved in intermolecular interactions, are omitted for clarity.
3. Materials and Methods
Silver(I) oxide (Aldrich, Milwaukee, WI, USA) was used as supplied. [(η5-C5Me5)IrCl(μ-Cl)]2 [16] and 1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazolium bromide [14] were prepared as previously described. The mass spectrum was recorded on a Bruker Daltonics micrOTOF spectrometer (Bilerica, MA, USA). The 1H and 19F NMR spectra were recorded using a Bruker DPX400 spectrometer (Bilerica, MA, USA). 1H was referenced internally using the residual protio solvent resonance relative to SiMe4 (δ 0), and 19F externally to CFCl3 (δ 0). All chemical shifts are quoted in δ (ppm), using the high frequency positive convention, and coupling constants in Hz.
3.1. Synthesis of (η5-Pentamethylcyclopentadienyl)iridium{1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazolin-2-ylidene}dichloride (2)
A mixture of silver(I) oxide (0.16 g, 0.69 mmol) and 1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazolium bromide (0.50 g, 1.16 mmol) in dichloromethane (10 cm3) was stirred in the absence of light. After 6 h, the mixture was twice filtered through celite, followed by removal of the solvent from the filtrate by rotary evaporation to afford {1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazolin-2-ylidene}silver bromide as a black solid. A portion of this (0.25 g, 0.40 mmol) was added to [(η5-C5Me5)IrCl(μ-Cl)]2 (0.17 g, 0.21 mmol) in dichloromethane (50 cm3) in the absence of light. After 16 h the reaction mixture was filtered through celite and the solvent removed under reduced pressure. Recrystallization from dichloromethane afforded the product as a yellow crystalline solid. Yield 0.26 g (79%). Anal. Calc. for C27H21Cl2F10IrN2.CH2Cl2: C, 36.90; H, 2.54; N, 3.07. Found C, 36.39; H, 2.58; N, 3.24%. MS(ESI): [M − Cl]+ found m/z = 790.8945; [C27H2135ClF10IrN2]+ requires m/z = 791.0936. 1H NMR (400.14 MHz, CDCl3): δ 6.52 (2H, s, HC=CH), 6.20 (2H, d, 2JH–H′ = 15.5 Hz, CHH′), 5.51 (2H, d, 2JH–H′ = 15.5 Hz, CHH′), 1.76 (15H, s, C5(CH3)5). 19F NMR (376.46 MHz, CDCl3): δ −140.39 (Fortho, dd, 3JF–F = 22.2 Hz, 8.0 Hz), −150.91 (Fpara, t, 3JF–F = 22.2 Hz), −159.64 (Fmeta, dt, 3JF–F = 22.2, 7.5 Hz) (A, M and X components of an AA′MXX′ spin system).
3.2. Single-Crystal XRD Determination
Crystals of 2 suitable for single-crystal X-ray diffraction were grown from chloroform. Diffraction data of a single crystal with dimensions 0.19 mm × 0.06 mm × 0.02 mm were collected at 100.0(1) K on an Agilent SuperNova (Santa Clara, CA, USA), single source at offset, Atlas diffractometer using with graphite-monochromated Cu—Kα radiation (λ = 1.54184 Å). The index ranges are: –18 ≤ h ≤ 15, –11 ≤ k ≤ 12, –25 ≤ l ≤ 23. A total of 9716 reflections were collected in the range θ 4.34° to 73.97°. There are 3239 independent reflections (Rint = 0.0386, Rsigma = 0.0372), of which 2825 are observable reflections with I > 2σ(I). Using Olex2 [17], the structure was solved with the Olex2.solve [18] structure programme using Charge Flipping and refined with the Olex2.refine [18] refinement package using Gauss–Newton minimization. The non-hydrogen atoms were refined with anisotropic thermal parameters. Hydrogen atom positions were added in idealized positions and a riding model with fixed thermal parameters (Uij = 1.2Ueq for the atom to which they are bonded (1.5 for CH3)) was used for subsequent refinements. The function minimized was Σ[w (|Fo|2 − |Fc|2)]. Crystal Data for C28H22Cl5F10IrN2 (M = 945.96 g/mol): orthorhombic, space group Pnam (no. 62), a = 15.1950(3) Å, b = 10.4051(3) Å, c = 20.3737(5) Å, V = 3221.22(12) Å3, Z = 4, μ(CuKα) = 12.599 mm−1, Dcalc = 1.950 g/cm3. The final R1 was 0.0338 (I > 2σ(I)) and wR2 was 0.0873 (all data).
Supplementary Materials
The following supporting information can be downloaded: Figures S1 and S2: 1H NMR spectrum of (η5-pentamethylcyclopentadienyl)iridium{1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazolin-2-ylidene}dichloride (2). Figure S3: 19F NMR spectrum of (η5-pentamethylcyclopentadienyl)iridium{1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazolin-2-ylidene}dichloride (2). Figures S4 and S5: Positive ion electrospray mass spectrum of (η5-pentamethylcyclopentadienyl)iridium{1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazolin-2-ylidene}dichloride (2).
Author Contributions
G.C.S. supervision, conceptualization, formal analysis and writing. A.C.M. conceptualization. S.K.B. synthesis, crystal growth and characterization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
CDCC No: 2234631 contains the supplementary crystallographic data for the title compound. The data can be obtained free of charge via http://www.ccdc.cam.ac.uk (accessed on 28 April 2023) from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: (+44)-1223-336-033; or via email: deposit@ccdc.cam.ac.uk.
Acknowledgments
The authors thank J. Burrows for the technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Rufino-Felipe, E.; Valdés, H.; Germán-Acacio, J.M.; Reyes-Márquez, V.; Morales-Morales, D. Fluorinated N-Heterocyclic carbene complexes. Applications in catalysis. J. Organomet. Chem. 2020, 921, 121364. [Google Scholar] [CrossRef]
- Marr, A.C.; Pollock, C.L.; Saunders, G.C. Base free dynamic kinetic resolution of secondary alcohols using ‘piano stool’ complexes of N-heterocyclic carbenes. Organometallics 2007, 26, 3283–3285. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Morgan, P.J.; Jackson, R.E.; Liu, X.; Saunders, G.C.; Lorenzini, F.; Marr, A.C. Designing effective homogeneous catalysis for glycerol valorisation: Selective synthesis of a value-added aldehyde from 1,3-propanediol via hydrogen transfer catalysed by a highly recyclable, fluorinated Cp*Ir(NHC) catalyst. Catal. Today 2018, 307, 248–259. [Google Scholar] [CrossRef]
- Pachal, S.R.; Saunders, G.C.; Weston, J.K. Complex Dependent Regioselectivity in the Nucleophilic Substitution of Fluorine of a Coordinated Tetrafluoropyridyl N-Heterocycle Carbene. Inorg. Chim. Acta 2013, 394, 558–562. [Google Scholar] [CrossRef]
- Thomas, H.P.; Marr, A.C.; Morgan, P.J.; Saunders, G.C. Tethering of Pentamethylcyclopentadienyl and N-Heterocycle Stabilized Carbene Ligands by Intramolecular 1,4-Addition to a Polyfluorophenyl Substituent. Organometallics 2018, 37, 1339–1341. [Google Scholar] [CrossRef]
- Marr, A.C.; Morgan, P.J.; Saunders, G.C.; Thomas, H.P. Conversion of haloform to carbonate by iridium N-heterocyclic carbene complexes and silver(I) oxide. Dalton Trans. 2019, 48, 1947–1949. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.J.; Hanson-Heine, M.W.D.; Thomas, H.P.; Saunders, G.C.; Marr, A.C.; Licence, P. C–F Bond Activation of a Perfluorinated Ligand Leading to Nucleophilic Fluorination of an Organic Electrophile. Organometallics 2020, 39, 2116–2124. [Google Scholar] [CrossRef]
- Thomas, H.P.; Wang, Y.; Lorenzini, F.; Coles, S.J.; Horton, P.N.; Marr, A.C.; Saunders, G.C. Cyclometallation via Carbon-Fluorine Bond Activation induced by Silver Particles. Organometallics 2017, 36, 960–963. [Google Scholar] [CrossRef]
- Marr, A.C.; Saunders, G.C.; Thomas, H.P.; Wang, Y. Orthometallation in Iridium N-Heterocycle Stabilized Carbene Complexes via Carbon—Halogen and Carbon—Hydrogen Bond Activation induced by Silver Nanoparticles. Inorg. Chim. Acta 2019, 486, 1–7. [Google Scholar] [CrossRef]
- Aznarez, F.; Gao, W.-X.; Lin, Y.-J.; Hahn, F.E.; Jin, G.-X. Preparation of polynuclear NHC complexes by post-synthetic modification of half-sandwich rhodium and iridium complexes bearing C-azolato ligands. Dalton Trans. 2018, 47, 9442–9452. [Google Scholar] [CrossRef] [PubMed]
- Aznarez, F.; Iglesias, M.; Hepp, A.; Veit, B.; Sanz Miguel, P.J.; Oro, L.A.; Jin, G.-X.; Hahn, F.E. Iridium(III) Complexes Bearing Chelating Bis-NHC Ligands and Their Application in the Catalytic Reduction of Imines. Eur. J. Inorg. Chem. 2016, 2016, 4598–4603. [Google Scholar] [CrossRef]
- Aznarez, F.; Sanz Miguel, P.J.; Tan, T.T.Y.; Hahn, F.E. Preparation of Rhodium(III) Di-NHC Chelate Complexes Featuring Two Different NHC Donors via a Mild NaOAc-Assisted C–H Activation. Organometallics 2016, 35, 410–419. [Google Scholar] [CrossRef]
- Lockley, A.J.; Marr, A.C.; Saunders, G.C.; Thomas, H.P. Arene–polyfluoroarene π-π stacking between N-heterocyclic carbene ligands of pentamethylcyclopentadienyl group 9 metal complexes. J. Fluor. Chem. 2019, 220, 1–8. [Google Scholar] [CrossRef]
- Lampl, M.; Schlapp-Hackl, I.; Wurst, K.; Gelbrich, T.; Kopacka, H.; Muller, T.; Kreutz, C.; Naier, B.; Partl, G.J.; Kahlenberg, V.; et al. Synthetic and structural studies on pentafluorobenzylated imidazole systems. J. Fluor. Chem. 2019, 218, 51–62. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- White, C.; Yates, A.; Maitlis, P.M. (η5-Pentamethylcyclopentadienyl)rhodium and -iridium compounds. Inorg. Synth. 1992, 29, 228–234. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. 2015, A71, 59–75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).