9-Methoxynaphtho[1,2-b]benzofuran
Abstract
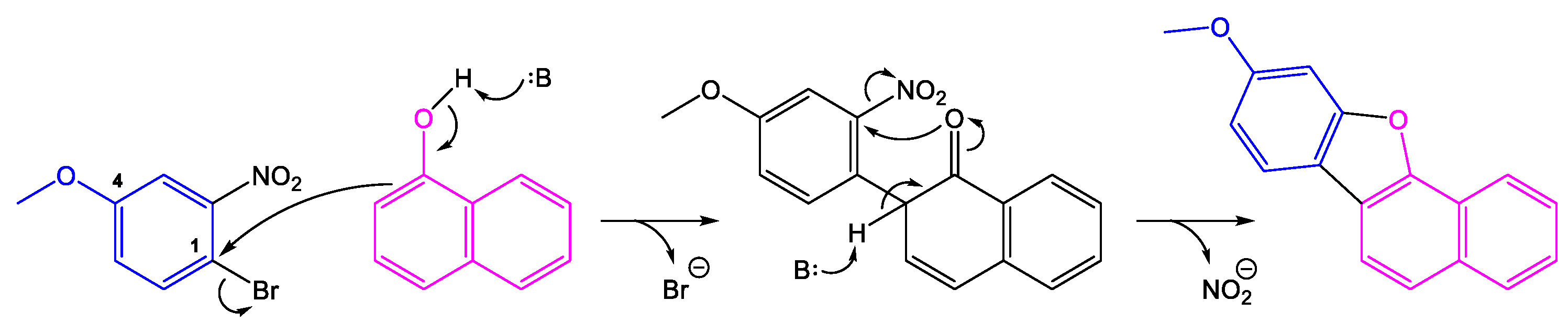
1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. General
3.2. Synthesis of 9-Methoxynaphtho[1,2-b]benzofuran
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, W.; Liu, Y.; Zhu, D. π-Conjugated Molecules with Fused Rings for Organic Field-Effect Transistors: Design, Synthesis and Applications. Chem. Soc. Rev. 2010, 39, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Aomori, S.; Oh-E, M. Synthesis, Properties and FET Characteristics of Oxygen-Based Heterocycle Dimers 9,9′-Bi(Benzo[b]Naphtha[2,1-d]Furan). Synth. Met. 2015, 209, 355–360. [Google Scholar] [CrossRef]
- Högberg, H.-E. Cyclo-oligomerization of Quinones. V. The Acid Catalyzed Reactions of alpha-Naphthoquinone with Phenols. Acta Chem. Scand. 1973, 27, 2559–2566. [Google Scholar] [CrossRef]
- Minami, Y.; Furuya, Y.; Hiyama, T. Facile Construction of Furanoacenes by a Three-Step Sequence Going through Disilyl-Exo-Cyclic Dienes. Chem. Eur. J. 2020, 26, 9471–9474. [Google Scholar] [CrossRef] [PubMed]
- Torres-Moya, I.; Harbuzaru, A.; Donoso, B.; Prieto, P.; Ortiz, R.P.; Díaz-Ortiz, Á. Microwave Irradiation as a Powerful Tool for the Preparation of N-Type Benzotriazole Semiconductors with Applications in Organic Field-Effect Transistors. Molecules 2022, 27, 4340. [Google Scholar] [CrossRef]
- Downie, I.M.; Heaney, H.; Kemp, G.; King, D.; Wosley, M. Cylisation Reactions of 2-Substituted Biphenyl-2′-Yldiazonium Salts Leading to O-Alkyldibenzofuranium and S-Alkyl-Dibenzothiophenium Salts: Modified Meerwein Reagents. Tetrahedron 1992, 48, 4005–4016. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, A.; Verma, A.; Jana, S.; Sattar, M.; Kumar, S.; Prasad, C.D.; Kumar, S. Chemoselective Arylation of Phenols with Bromo-Nitroarenes: Synthesis of Nitro-Biaryl-Ols and Their Conversion into Benzofurans and Carbazoles. Chem. Comm. 2014, 50, 9481–9484. [Google Scholar] [CrossRef] [PubMed]
- Bunnett, J.F.; Zahlerz, R.E. Aromatic Nucleophilic Substitution Reactions. Chem. Rev. 1951, 49, 273–412. [Google Scholar] [CrossRef]
- Crampton, M.R. Nucleophilic Aromatic. Substitution. Arene Chem. 2015, 131–173. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miani, R.; Vigna, J.; Mancini, I. 9-Methoxynaphtho[1,2-b]benzofuran. Molbank 2023, 2023, M1632. https://doi.org/10.3390/M1632
Miani R, Vigna J, Mancini I. 9-Methoxynaphtho[1,2-b]benzofuran. Molbank. 2023; 2023(2):M1632. https://doi.org/10.3390/M1632
Chicago/Turabian StyleMiani, Roberto, Jacopo Vigna, and Ines Mancini. 2023. "9-Methoxynaphtho[1,2-b]benzofuran" Molbank 2023, no. 2: M1632. https://doi.org/10.3390/M1632
APA StyleMiani, R., Vigna, J., & Mancini, I. (2023). 9-Methoxynaphtho[1,2-b]benzofuran. Molbank, 2023(2), M1632. https://doi.org/10.3390/M1632






