Abstract
In continuation of our research program to develop original synthetic methods using TDAE methodology on nitroheterocyclic substrates, we were able to generate for the first time a stable carbanion in position 2 of the 5-nitroimidazole scaffold. Starting from a 2-chloromethyl-4-phenylsulfonylmethyl-5-nitroimidazole intermediate, prepared by the vicarious nucleophilic substitution of hydrogen (VNS) reaction, we selectively introduced a N-tosylbenzylimine moiety at position 2 without reducing the sulfone at position 4, leading to the formation of N-[1-(2-chlorophenyl)-2-{1-methyl-5-nitro-4-[(phenylsulfonyl)methyl]-1H-imidazol-2-yl}ethyl]-4-methylbenzenesulfonamide in 47% yield. This new synthetic method using TDAE should allow access to new 2-substituted 5-nitroimidazole derivatives with various electrophiles such as carbonyls and other N-tosylbenzylimines.
1. Introduction
5-Nitroimidazoles are historically known for their antibacterial and anti-parasitic properties. Discovered in the 1960s [1], metronidazole (Figure 1) is still widely used for the treatment of parasitic infections caused by Entamoeba histolytica and Giardia lamblia [2] as well as infections caused by anaerobic bacteria such as Bacteroides, Clostridioides or Helicobacter pylori [3,4]. In 2019, fexinidazole (Figure 1) received marketing authorization as the first all-oral treatment against Trypanosoma brucei gambiense [5], renewing interest in the development of new nitroheterocycles active against kinetoplastid infections, such as leishmaniases, sleeping sickness and Chagas disease. Thus, the development of original synthetic methods allowing the access to new 5-nitroimidazole derivatives is likely to be of interest for medicinal chemistry.
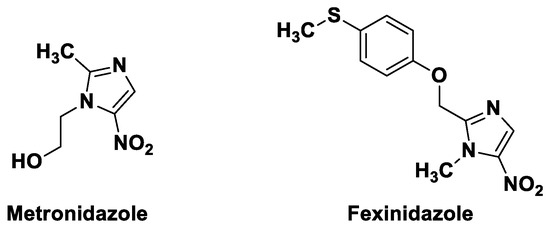
Figure 1.
Structures of metronidazole and fexinidazole.
Tetrakis(dimethylamino)ethylene (TDAE) is an organic reducing agent that has the specific property of activating the carbon-halogen bond to generate a carbanion [6]. Since 2002, our team has developed several reactions between nitroheterocyclic substrates and various electrophiles such as carbonyls and N-tosylbenzylimines using the TDAE methodology [7]. This strategy led to the synthesis of new 1,2-dimethyl-5-nitro-1H-imidazole derivatives bearing a phenyl or styryl group in position 4 (Scheme 1) [8,9].
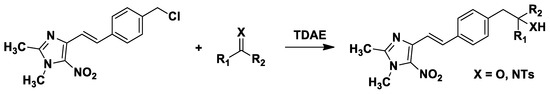
Scheme 1.
Preparation of 4-(4-substituted)styryl-1,2-dimethyl-5-nitro-1H-imidazole derivatives using TDAE.
Based on previous work focused on the study of the vicarious nucleophilic substitution of hydrogen (VNS) reaction on the 5-nitroimidazole scaffold [10], our team also reported the synthesis of 2,4-disubstituted 5-nitroimidazole compounds using the VNS reaction to introduce a phenylsulfonylmethyl substituent at position 4. Some compounds showed potent antibacterial activity against Clostridioides difficile [11], while other compounds showed better antiparasitic activity than metronidazole and were associated with lower mutagenicity [12].
In continuation of this research program, we were able to generate for the first time a stable carbanion at position 2 of the 5-nitroimidazole scaffold using TDAE, which allowed us to introduce an N-tosylbenzylimine moiety at position 2 without reducing the sulfone at position 4.
2. Results
The 2-chloromethyl-4-phenylsulfonylmethyl-5-nitroimidazole intermediate (2) was obtained by vicarious nucleophilic substitution of hydrogen at position 4 of commercially available (1-methyl-5-nitro-1H-imidazol-2-yl)methanol (Scheme 2), followed by chlorination at position 2 with thionyl chloride [13,14].
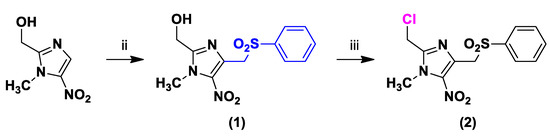
Scheme 2.
Synthesis of compounds 1 and 2.
Reagents and conditions: (ii) Chloromethylsulfonylbenzene 2 equiv, tBuOK 4 equiv, DMF, −40 °C, 15 min, 40%; (iii) Thionyl chloride 2 equiv, CH2Cl2, 4 h, 67%.
Finally, the reaction of intermediate 2 with 1.5 equivalents of (E)-N-(2-chlorobenzylidene)-4-methylbenzenesulfonamide [15] in the presence of TDAE at −20 °C for 1 hour led to the desired compound 3 in moderate yield (Scheme 3).
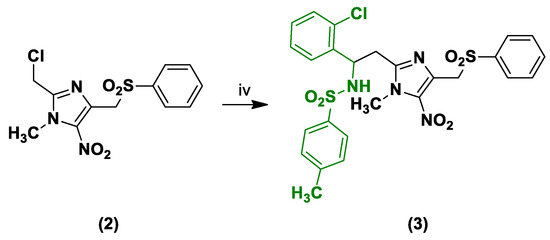
Scheme 3.
Synthesis of compound 3.
Reagents and conditions: (iv) (E)-N-(2-Chlorobenzylidene)-4-methylbenzenesulfonamide 1.5 equiv, TDAE 1 equiv, DMF, N2, −20 °C, 1 h, 47%.
3. Discussion
Using our knowledge about the reactivity of activated chloromethyl groups in TDAE methodologies, we determined that the chloromethyl group at position 2 of a 4-phenylsulfonylmethyl-5-nitroimidazole derivative could lead to a stable carbanion. Indeed, the electron withdrawing effect (-M) of the nitro group at position 5 promotes the activation of the carbon-halogen bond of the chloromethyl group in position 2 and the stabilization of the carbanion produced by TDAE.
This study reports on the first time that a stable carbanion has been generated at position 2 of the 5-nitroimidazole scaffold. Moreover, mild conditions of the TDAE strategy allowed us to specifically activate the carbon-chlorine bond at position 2 without reducing the sulfone at position 4. This led to the introduction of a N-tosylbenzylimine moiety at position 2 and the synthesis of compound 3 in moderate yield.
The development of this new synthetic method using TDAE should allow access to new 2-substituted 5-nitroimidazole derivatives with various electrophiles such as carbonyls and other N-tosylbenzylimines.
4. Materials and Methods
Melting points were determined on a Köfler melting point apparatus (Wagner & Munz GmbH, München, Germany) and were uncorrected. HRMS spectrum (ESI) was recorded on a SYNAPT G2 HDMS (Waters) at the Faculté des Sciences de Saint-Jérôme (Marseille). NMR spectra were recorded on a Bruker Avance 200 MHz or a Bruker Avance NEO 400 MHz NanoBay spectrometer at the Faculté de Pharmacie of Marseille. (1H NMR: reference CDCl3 δ = 7.26 ppm, reference DMSO-d6 δ = 2.50 ppm and 13C NMR: reference CDCl3 δ = 76.9 ppm, reference DMSO-d6 δ = 39.52 ppm). The following adsorbent was used for column chromatography: silica gel 60 (Merck KGaA, Darmstadt, Germany, particle size 0.063–0.200 mm, 70–230 mesh ASTM). TLC was performed on 5 cm × 10 cm aluminum plates coated with silica gel 60F-254 (Merck) in an appropriate eluent. Visualization was performed with ultraviolet light (234 nm). The purity determination of synthesized compounds was checked by LC/MS analyses, which were realized at the Faculté de Pharmacie of Marseille with a Thermo Scientific Accela High-Speed LC System® (Waltham, MA, USA) coupled using a single quadrupole mass spectrometer Thermo MSQ Plus®. The purity of synthesized compound 3 was >95%. The RP-HPLC column is a Thermo Hypersil Gold® 50 × 2.1 mm (C18 bounded), with particles of a diameter of 1.9 mm. The volume of the sample injected into the column was 1 µL. Chromatographic analysis, total duration of 8 min, was on the gradient of the following solvents: t = 0 min, methanol/water 50:50; 0 < t < 4 min, linear increase in the proportion of methanol to a methanol/water ratio of 95:5; 4 < t < 6 min, methanol/water 95:5; 6 < t < 7 min, linear decrease in the proportion of methanol to return to a methanol/water ratio of 50:50; 6 < t < 7 min, methanol/water 50:50. The water used was buffered with ammonium acetate 5 mM. The flow rate of the mobile phase was 0.3 mL/min. The retention times (tR) of the molecules analyzed were indicated in min. Reagents were purchased from Sigma-Aldrich or Fluorochem and used without further purification. Copies of 1H NMR and 13C NMR are available in the Supplementary Materials.
4.1. {1-Methyl-5-nitro-4-[(phenylsulfonyl)methyl]-1H-imidazol-2-yl}methanol (1)
To a solution of tBuOK (1.95 g, 17.4 mmol, 4 equiv) in dry DMF (15 mL) at −40 °C was added a solution of chloromethylsulfonylbenzene (1.66 g, 8.7 mmol, 2 equiv) in DMF (15 mL). Then, a solution of (1-methyl-5-nitro-1H-imidazol-2-yl)methanol (0.683 g, 4.35 mmol, 1 equiv.) was added dropwise and the reaction mixture was stirred at −40 °C for 15 min. A 2 M solution of HCl was added until pH 1 and the aqueous layer was extracted three times with ethyl acetate. Then, the organic layer was washed four times with brine, dried over anhydrous Na2SO4, filtered and evaporated. Compound 1 was obtained after purification by chromatography on silica gel (eluent: cyclohexane-dichloromethane 2:8) as a yellow solid in 40% yield (0.541 g). mp 153 °C. 1H NMR (250 MHz, DMSO-d6) δ: 7.75–7.72 (m, 3H), 7.64–7.58 (m, 2H), 5.74 (brs, 1H), 4.91 (s, 2H), 4.53 (s, 2H), 3.87 (s, 3H). 13C NMR 63 MHz, DMSO-d6) δ: 150.4, 138.9, 137.4, 134.2, 131.6, 129.4 (2C), 127.9 (2C), 55.9, 55.3, 34.1. LC/MS ESI+ tR 3.17, (m/z) [M + H]+ 312.07. HRMS (+ESI): 312.0646 [M + H]+. Calcd for C12H14N3O5S: 312.0649.
4.2. 2-(Chloromethyl)-1-methyl-5-nitro-4-[(phenylsulfonyl)methyl]-1H-imidazole (2)
To a solution of {1-methyl-5-nitro-4-[(phenylsulfonyl)methyl]-1H-imidazol-2-yl}methanol (1) (0.5 g, 1.61 mmol, 1 equiv) in dichloromethane (15 mL) at 0 °C was added thionyl chloride (234 µL, 3.22 mmol, 2 equiv) and the reaction mixture was stirred at room temperature for 4 h. A 1 M K2CO3 solution was added to neutralize and the aqueous layer was extracted three times with dichloromethane. Then, the organic layer was washed four times with brine, dried over anhydrous Na2SO4, filtered and evaporated. Compound 2 was obtained after purification by chromatography on silica gel (eluent: petroleum ether-ethyl acetate 5:5) as a brown solid in 67% yield (0.354 g). mp 154 °C. 1H NMR (250 MHz, CDCl3) δ: 7.87–7.75 (m, 2H), 7.70–7.59 (m, 1H), 7.55–7.49 (m, 2H), 4.82 (s, 2H), 4.63 (s, 2H), 3.97 (s, 3H). 13C NMR 63 MHz, CDCl3) δ: 145.9, 138.8, 137.9, 134.3, 132.4, 129.3 (2C), 128.4 (2C), 55.9, 40.0, 34.7. LC/MS ESI+ tR 4.28, (m/z) [M + H]+ 330.05/332.04. HRMS (+ESI): 330.0311 [M + H]+. Calcd for C12H13ClN3O4S: 330.0310.
4.3. N-[1-(2-Chlorophenyl)-2-{1-methyl-5-nitro-4-[(phenylsulfonyl)methyl]-1H-imidazol-2-yl}ethyl]-4-methylbenzenesulfonamide (3)
To a solution of 2-(chloromethyl)-1-methyl-5-nitro-4-[(phenylsulfonyl)methyl]-1H-imidazole (2) (0.2 g, 0.61 mmol, 1 equiv.) in dry DMF (7 mL) at −20 °C was added (E)-N-(2-chlorobenzylidene)-4-methylbenzenesulfonamide (0.27 g, 0.92 mmol, 1.5 equiv). Then, tetrakis(dimethylamino)ethylene (142 µL, 0.61 mmol, 1 equiv) was added under N2 atmosphere and the reaction mixture was stirred at −20 °C for 1 h. The mixture was slowly poured into an ice-water mixture, extracted three times with ethyl acetate. Then, the organic layer was washed four times with brine, dried over anhydrous Na2SO4, filtered and evaporated. Compound 3 was obtained after purification by chromatography on silica gel (eluent: dichloromethane) as a beige solid in 47% yield (0.17 g). mp 136 °C. 1H NMR (400 MHz, DMSO-d6) δ: 8.67 (d, J = 9.4 Hz, 1H), 7.79–7.66 (m, 3H), 7.66–7.55 (m, 3H), 7.39–7.33 (m, 3H), 7.33–7.21 (m, 2H), 7.14 (d, J = 8.1 Hz, 2H), 5.03 (ddd, J = 9.9, 9.4, 5.0 Hz, 1H), 4.80 (s, 2H), 3.64 (s, 3H), 2.99 (dd, J = 15.1, 9.9 Hz, 1H), 2.93 (dd, J = 15.1, 5.0 Hz, 1H), 2.26 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ: 147.9, 142.3, 138.8, 138.0, 136.5, 134.0, 132.7, 130.9, 129.2 (3C), 129.1 (2C), 129.0 (2C), 128.2, 128.0 (2C), 127.6, 126.0 (2C), 55.3, 52.7, 34.0, 33.9, 20.8. LC/MS ESI+ tR 5.77, (m/z) [M + H]+ 588.82/590.85. HRMS (+ESI): 589.0978 [M + H]+. Calcd for C26H26ClN4O6S2: 589.0977.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1: 1H NMR spectrum of compound 3. Figure S2: 13C NMR spectrum of compound 3.
Author Contributions
Conceptualization, N.P. and R.P.-L.; methodology, N.P.; validation, N.P.; formal analysis, R.P.-L.; investigation, R.P.-L. and I.J.; resources, P.V.; writing—original draft preparation, R.P.-L.; writing—review and editing, N.P., P.V., P.R. and C.C.-D.; supervision, N.P., P.V. and P.R.; project administration, N.P. and P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Aix-Marseille Université (AMU)”, by “Centre national de la recherche scientifique (CNRS)” and by “Assistance publique-Hôpitaux de Marseille (AP-HM)”.
Data Availability Statement
Not applicable.
Acknowledgments
We want to thank Vincent Remusat (Institut de Chimie Radicalaire, Marseille) for his help with NMR analysis, Valérie Monnier and Gaëlle Hisler (Spectropole, Marseille) for performing HRMS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Durel, P.; Couture, J.; Collart, P.; Girot, C. Flagyl (Metronidazole). Sex. Transm. Infect. 1960, 36, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Metronidazole to Clear Intestinal Parasites. Lancet 1996, 348, 273. [Google Scholar] [CrossRef] [PubMed]
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Metronidazole in Anaerobic Infections: A Review of Its Activity, Pharmacokinetics and Therapeutic Use. Drugs 1978, 16, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Löfmark, S.; Edlund, C.; Nord, C.E. Metronidazole Is Still the Drug of Choice for Treatment of Anaerobic Infections. Clin. Infect. Dis. 2010, 50, S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Pelfrene, E.; Harvey Allchurch, M.; Ntamabyaliro, N.; Nambasa, V.; Ventura, F.V.; Nagercoil, N.; Cavaleri, M. The European Medicines Agency’s Scientific Opinion on Oral Fexinidazole for Human African Trypanosomiasis. PLoS Negl. Trop. Dis. 2019, 13, e0007381. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, C.; Dolbier, W.R.; Medebielle, M.; Ndedi, A. Tetrakis (Dimethylamino) Ethylene (TDAE) as a Useful Reductant of Some Chlorodifluoromethylated Ketones. A New Approach for the Synthesis of α,α-Difluoroketone Derivatives. Tetrahedron Lett. 1998, 39, 8853–8856. [Google Scholar] [CrossRef]
- Giuglio-Tonolo, G.; Terme, T.; Médebielle, M.; Vanelle, P. Original Reaction of P-Nitrobenzyl Chloride with Aldehydes Using Tetrakis (Dimethylamino) Ethylene (TDAE). Tetrahedron Lett. 2003, 44, 6433–6435. [Google Scholar] [CrossRef]
- Crozet, M.; Terme, T.; Vanelle, P. Designing New 5-Nitroimidazoles: Towards Safer Anti-Infectious Agents. Lett. Drug Des. Discov. 2013, 11, 531–559. [Google Scholar] [CrossRef]
- Primas, N.; Neildé, K.; Kabri, Y.; Crozet, M.; Terme, T.; Vanelle, P. Exploring the Synthesis and the Reactivity of 4-[4-(chloromethyl) Styryl]-1,2-Dimethyl-5-Nitro-1H-Imidazole in TDAE Strategy. Synthesis 2013, 46, 348–356. [Google Scholar] [CrossRef]
- Makosza, M.; Kwast, E. Vicarious Nucleophilic Substitution of Hydrogen in Imidazole Derivatives. Bull. Pol. Acad. Sci. Chem. 1987, 35, 287–292. [Google Scholar]
- Spitz, C.; Mathias, F.; Péchiné, S.; Doan, T.H.D.; Innocent, J.; Pellissier, S.; Di Giorgio, C.; Crozet, M.D.; Janoir, C.; Vanelle, P. 2,4-Disubstituted 5-Nitroimidazoles Potent against Clostridium Difficile. ChemMedChem 2019, 14, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Crozet, M.D.; Botta, C.; Gasquet, M.; Curti, C.; Rémusat, V.; Hutter, S.; Chapelle, O.; Azas, N.; De Méo, M.; Vanelle, P. Lowering of 5-Nitroimidazole’s Mutagenicity: Towards Optimal Antiparasitic Pharmacophore. Eur. J. Med. Chem. 2009, 44, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Makosza, M.; Winiarski, J. Vicarious Nucleophilic Substitution of Hydrogen. Acc. Chem. Res. 1987, 20, 282–289. [Google Scholar] [CrossRef]
- Makosza, M.; Wojciechowski, K. Nucleophilic Substitution of Hydrogen in Heterocyclic Chemistry. Chem. Rev. 2004, 104, 2631–2666. [Google Scholar] [CrossRef] [PubMed]
- Sharghi, H.; Hosseini-Sarvari, M.; Ebrahimpourmoghaddam, S. A Novel Method for the Synthesis of N-Sulfonyl Aldimines Using AlCl3 under Solvent-Free Conditions (SFC). Arkivoc 2007, 2007, 255–264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).