Manganese(II) Bromide Coordination toward the Target Product and By-Product of the Condensation Reaction between 2-Picolylamine and Acenaphthenequinone
Abstract
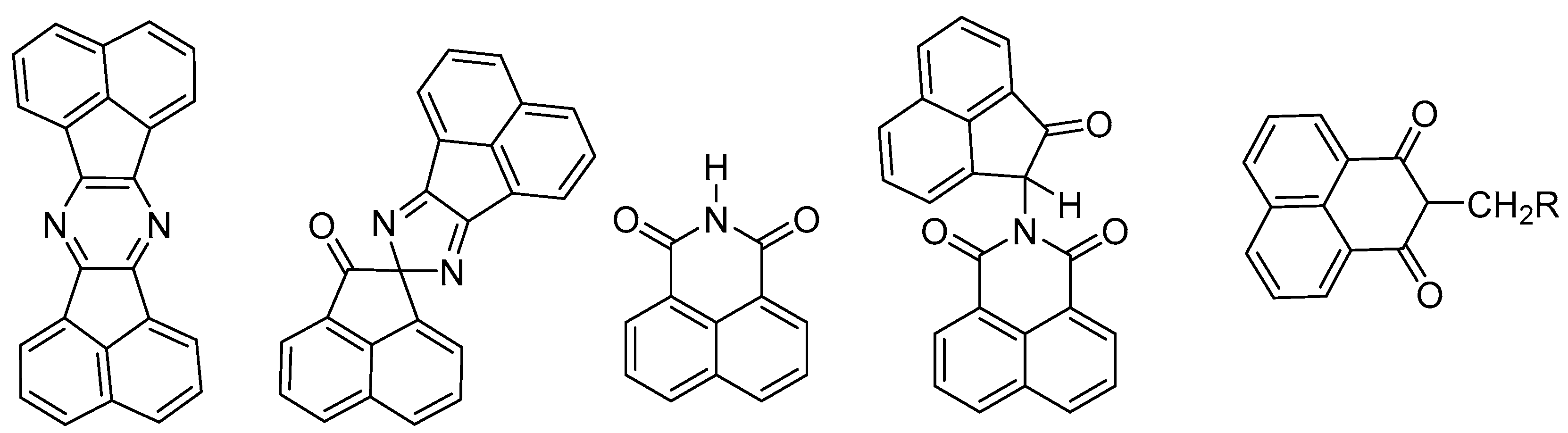
1. Introduction
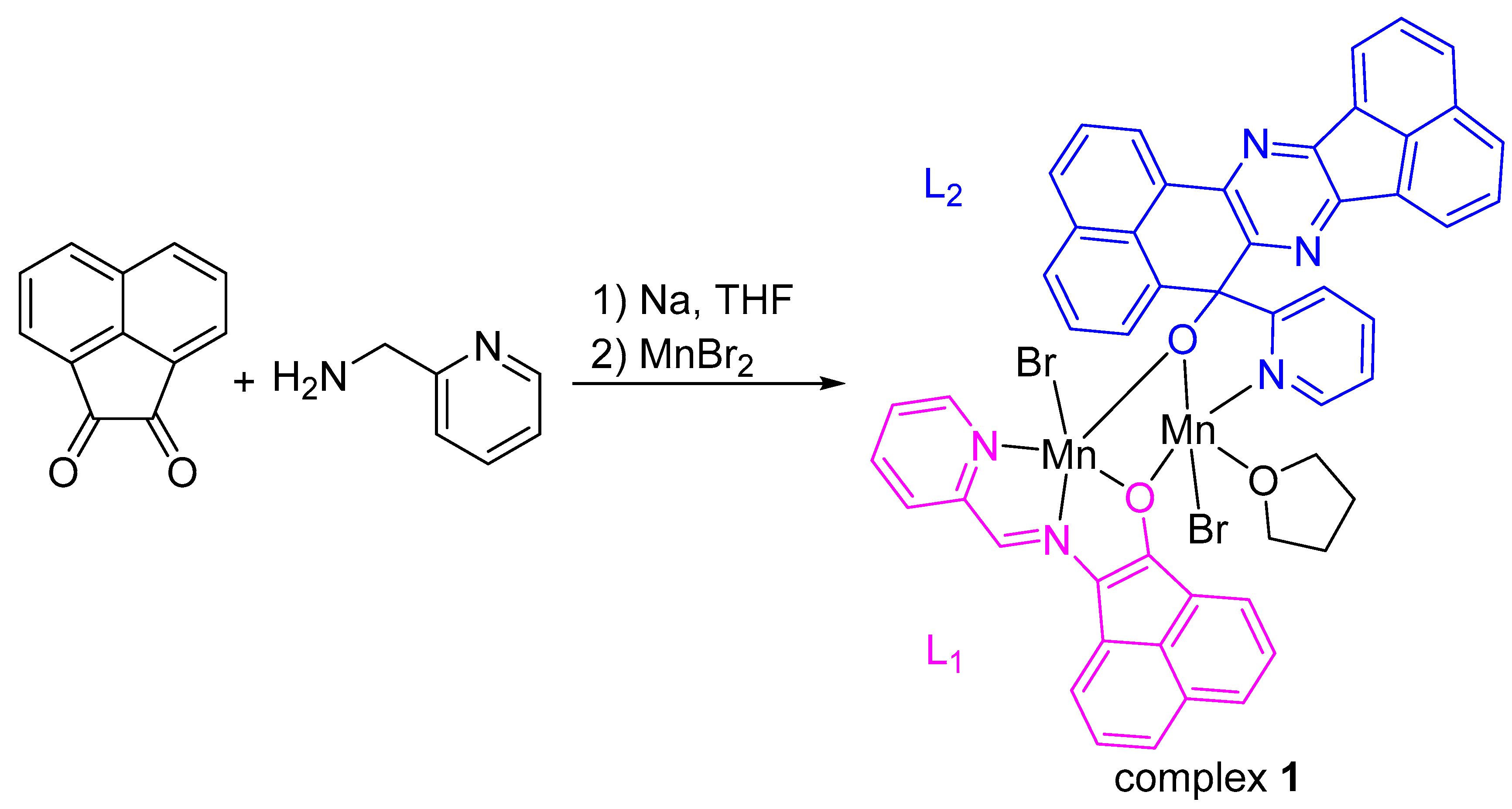
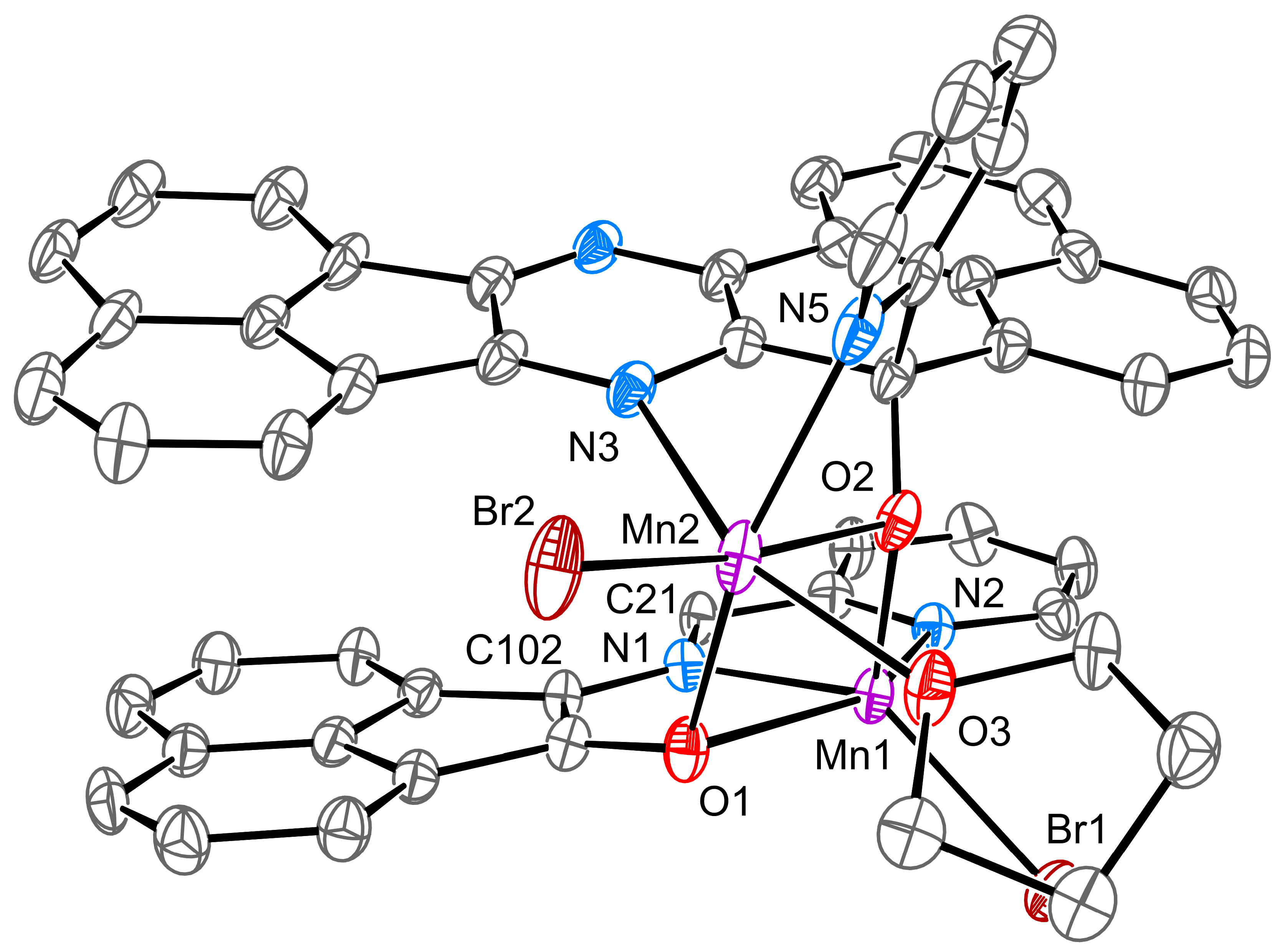
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gasperini, M.; Ragaini, F.; Cenini, S. Synthesis of Ar-BIAN Ligands (Ar-BIAN = Bis(aryl)acenaphthenequinonediimine) Having Strong Electron-Withdrawing Substituents on the Aryl Rings and Their Relative Coordination Strength toward Palladium(0) and -(II) Complexes. Organometallics 2002, 21, 2950–2957. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Kazarina, O.V.; Lukoyanov, A.N.; Skatova, A.A.; Bazyakina, N.L.; Cherkasov, A.V.; Palamidis, E. Mononuclear dpp-Bian Gallium Complexes: Synthesis, Crystal Structures, and Reactivity toward Alkynes and Enones. Organometallics 2015, 34, 1498–1506. [Google Scholar] [CrossRef]
- Gottumukkala, A.L.; Teichert, J.F.; Heijnen, D.; Eisink, N.; Dijk, S.; Ferrer, C.; Hoogenband, A.; Minnaard, A.J. Pd-Diimine: A Highly Selective Catalyst System for the Base-Free Oxidative Heck Reaction. J. Org. Chem. 2011, 76, 3498–3501. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.; Miesel, D.; Hildebrandt, A.; Ragaini, F.; Schaarschmidt, D.; Wangelin, A.J. Synthesis and Catalysis of Redox-Active Bis(imino)acenaphthene (BIAN) Iron Complexes. ChemCatChem 2017, 9, 3203–3209. [Google Scholar] [CrossRef]
- Hazari, A.S.; Ray, R.; Hoque, M.A.; Lahiri, G.K. Electronic Structure and Multicatalytic Features of Redox-Active Bis(arylimino)acenaphthene (BIAN)-Derived Ruthenium Complexes. Inorg. Chem. 2016, 55, 8160–8173. [Google Scholar] [CrossRef] [PubMed]
- Soshnikov, I.E.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.-H.; Talsi, E.P. Ethylene Polymerization of Nickel Catalysts with α-Diimine Ligands: Factors Controlling the Structure of Active Species and Polymer Properties. Dalton Trans. 2019, 48, 7974–7984. [Google Scholar] [CrossRef] [PubMed]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Kiskin, M.A.; Magdesieva, T.V.; Levitskiy, O.A.; Korchagin, D.V.; Efimov, N.N.; Vasil’ev, P.N.; Goloveshkin, A.S.; Sidorov, A.A.; et al. Complexes of Cobalt(II) Iodide with Pyridine and Redox Active 1,2-Bis(arylimino)acenaphthene: Synthesis, Structure, Electrochemical, and Single Ion Magnet Properties. Molecules 2020, 25, 2054. [Google Scholar] [CrossRef] [PubMed]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Kiskin, M.A.; Kholin, K.V.; Khrizanforov, M.N.; Yu, G.; Babeshkin, K.A.; Efimov, N.N.; Goloveshkin, A.S.; Imshennik, V.K.; et al. Generation of a Hetero Spin Complex from Iron(II) Iodide with Redox Active Acenaphthene-1,2-Diimine. Molecules 2021, 26, 2998. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.A.; Janetzki, J.T.; Kumar, V.J.; Gable, R.W.; Clérac, R.; Starikova, A.A.; Low, P.J.; Boskovic, C. Modulation of Charge Distribution in Cobalt-α-Diimine Complexes toward Valence Tautomerism. Inorg. Chem. 2022, 61, 17609–17622. [Google Scholar] [CrossRef] [PubMed]
- Kaim, V.; Kaur-Ghumaan, S. Manganese Complexes: Hydrogen Generation and Oxidation. Eur. J. Inorg. Chem. 2019, 2019, 5041–5051. [Google Scholar] [CrossRef]
- Moore, J.A.; Vasudevan, K.; Hill, N.J.; Reeske, G.; Cowley, A.H. Facile routes to Alkyl-BIAN ligands. Chem. Commun. 2006, 27, 2913–2915. [Google Scholar] [CrossRef] [PubMed]
- Ragaini, F.; Gasperini, M.; Parma, P.; Gallo, E.; Casati, N.; Macchi, P. Stability-inducing strain: Application to the synthesis of alkyl-BIAN ligands (alkyl-BIAN = bis(alkyl)acenaphthenequinonediimine). New J. Chem. 2006, 30, 1046–1057. [Google Scholar] [CrossRef]
- Ragaini, F.; Gasperini, M.; Gallo, E.; Macchi, P. Using ring strain to inhibit a decomposition path: First synthesis of an Alkyl-BIAN ligand (Alkyl-BIAN = bis(alkyl)acenaphthenequinonediimine). Chem. Commun. 2005, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Ragaini, F.; Monticelli, E.; Caselli, A.; Macchi, P.; Casati, N. Chiral cyclopropylamines in the synthesis of new ligands; first asymmetric Alkyl-BIAN compounds. Chem. Commun. 2010, 46, 6153–6155. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, O.; Tashiro, M. Studies of Acenaphthene Derivatives. XI. The Reaction of Acenaphthenequinone with Aliphatic Amines. Bull. Chem. Soc. Jpn. 1965, 38, 399–402. [Google Scholar] [CrossRef]
- Tsuge, O.; Tashiro, M. Studies of Acenaphthene Derivatives. XII. On the Red Substance Obtained from Acenaphthenequinone and Ammonia. Bull. Chem. Soc. Jpn. 1966, 39, 2477–2479. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khrizanforova, V.V.; Fayzullin, R.R.; Budnikova, Y.H. Manganese(II) Bromide Coordination toward the Target Product and By-Product of the Condensation Reaction between 2-Picolylamine and Acenaphthenequinone. Molbank 2023, 2023, M1606. https://doi.org/10.3390/M1606
Khrizanforova VV, Fayzullin RR, Budnikova YH. Manganese(II) Bromide Coordination toward the Target Product and By-Product of the Condensation Reaction between 2-Picolylamine and Acenaphthenequinone. Molbank. 2023; 2023(1):M1606. https://doi.org/10.3390/M1606
Chicago/Turabian StyleKhrizanforova, Vera V., Robert R. Fayzullin, and Yulia H. Budnikova. 2023. "Manganese(II) Bromide Coordination toward the Target Product and By-Product of the Condensation Reaction between 2-Picolylamine and Acenaphthenequinone" Molbank 2023, no. 1: M1606. https://doi.org/10.3390/M1606
APA StyleKhrizanforova, V. V., Fayzullin, R. R., & Budnikova, Y. H. (2023). Manganese(II) Bromide Coordination toward the Target Product and By-Product of the Condensation Reaction between 2-Picolylamine and Acenaphthenequinone. Molbank, 2023(1), M1606. https://doi.org/10.3390/M1606








