Synthesis of 2-(5-(2-Aminopropyl)-2-hydroxyphenyl)acetic Acid, a Metabolite of the Drug 5-APB
Abstract
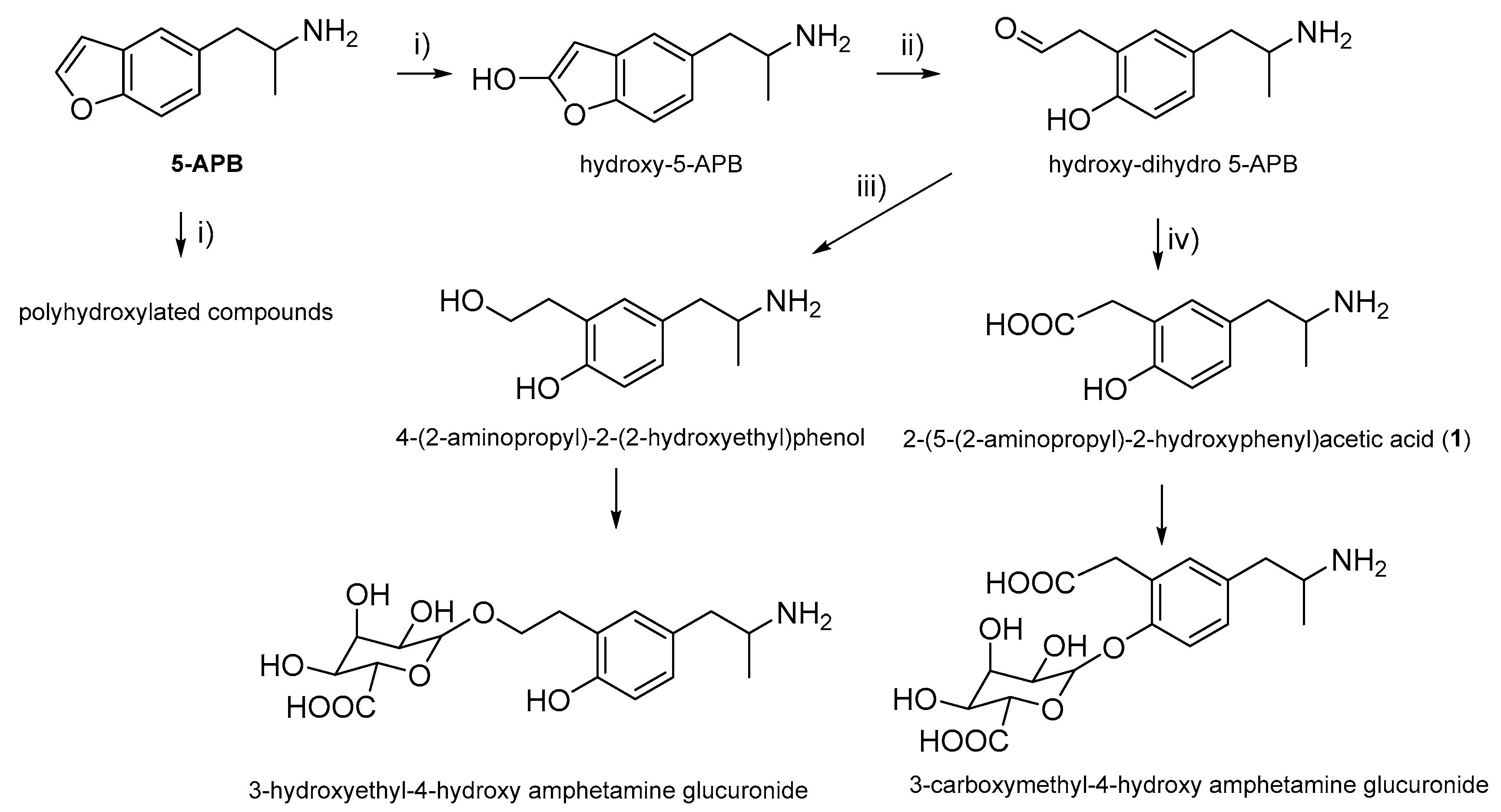
1. Introduction
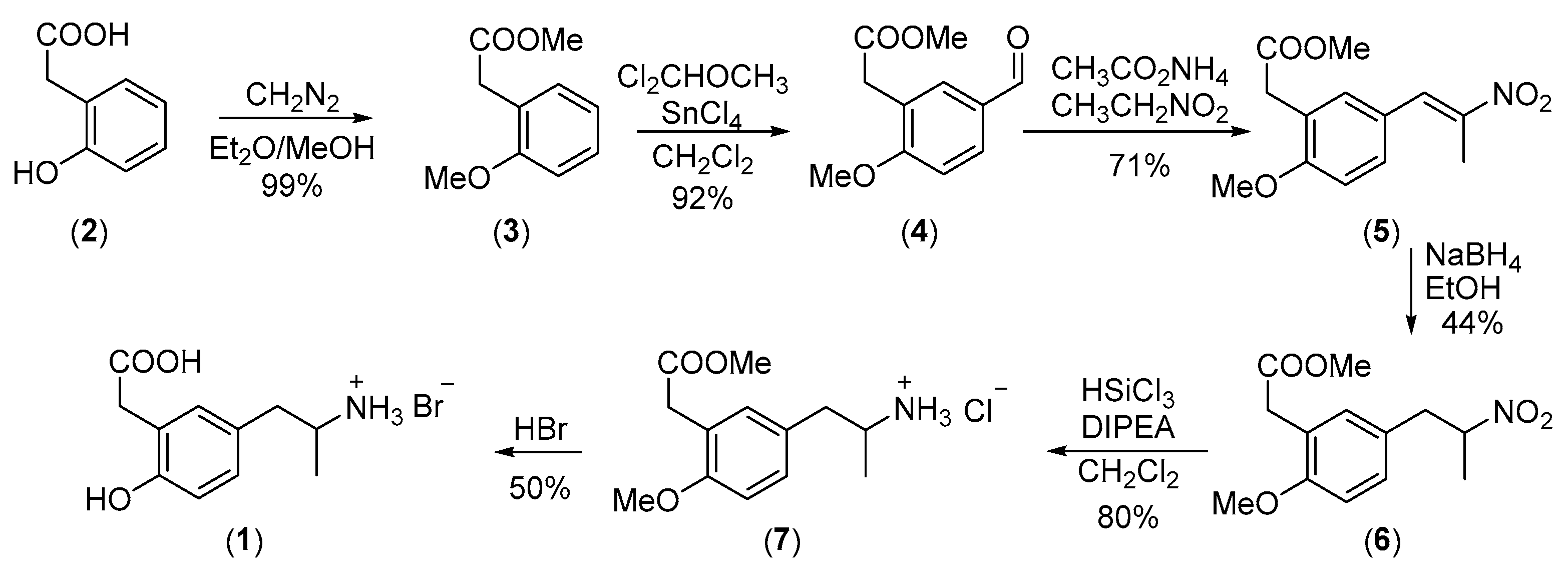
2. Results and Discussion
3. Materials and Methods
3.1. Methyl 2-(2-Methoxyphenyl)acetate (3)
3.2. Methyl 2-(5-Formyl-2-methoxyphenyl)acetate (4)
3.3. Methyl (E)-2-(2-Methoxy-5-(2-nitroprop-1-en-1-yl)phenyl)acetate (5)
3.4. Methyl 2-(2-Methoxy-5-(2-nitropropyl)phenyl)acetate (6)
3.5. Methyl 2-(5-(2-Aminopropyl)-2-methoxyphenyl)acetate Hydrochloride (7)
3.6. 2-(5-(2-Aminopropyl)-2-hydroxyphenyl)acetic Acid Hydrobromide (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zawilska, J.B.; Andrzejczak, D. Next Generation of Novel Psychoactive Substances on the Horizon—A Complex Problem to Face. Drug Alcohol Depend. 2015, 157, 1–17. [Google Scholar] [CrossRef]
- Monte, A.P.; Maronalewicka, D.; Cozzi, N.V.; Nichols, D.E. Synthesis and Pharmacological Examination of Benzofuran, Indan, and Tetralin Analogs of 3,4-(Methylenedioxy)Amphetamine. J. Med. Chem. 1993, 36, 3700–3706. [Google Scholar] [CrossRef]
- Bravo, R.R.; Carmo, H.; Carvalho, F.; Bastos, M.D.; da Silva, D.D. Benzo Fury: A New Trend in the Drug Misuse Scene. J. Appl. Toxicol. 2019, 39, 1083–1095. [Google Scholar] [CrossRef]
- Roque Bravo, R.; Silva, J.P.; Carmo, H.; Carvalho, F.; Dias da Silva, D. The Toll of Benzofurans in the Context of Drug Abuse. In Handbook of Substance Misuse and Addictions; Patel, V.B., Preedy, V.R., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Turcant, A.; Deguigne, M.; Ferec, S.; Bruneau, C.; Leborgne, I.; Lelievre, B.; Gegu, C.; Jegou, F.; Abbara, C.; Le Roux, G.; et al. A 6-Year Review of New Psychoactive Substances at the Centre Antipoison Grand-Ouest D’angers: Clinical and Biological Data. Toxicol. Anal. Clin. 2017, 29, 18–33. [Google Scholar] [CrossRef]
- Barcelo, B.; Gomila, I.; Rotolo, M.C.; Marchei, E.; Kyriakou, C.; Pichini, S.; Roset, C.; Elorza, M.A.; Busaro, F.P. Intoxication Caused by New Psychostimulants: Analytical Methods to Disclose Acute and Chronic Use of Benzofurans and Ethylphenidate. Int. J. Legal Med. 2017, 131, 1543–1553. [Google Scholar] [CrossRef]
- Deville, M.; Dubois, N.; Cieckiewicz, E.; De Tullio, P.; Lemaire, E.; Charlier, C. Death Following Consumption of Mdai and 5-Eapb. Forensic Sci. Int. 2019, 299, 89–94. [Google Scholar] [CrossRef]
- McIntyre, I.M.; Gary, R.D.; Trochta, A.; Stolberg, S.; Stabley, R. Acute 5-(2-Aminopropyl)Benzofuran (5-Apb) Intoxication and Fatality: A Case Report with Postmortem Concentrations. J. Anal. Toxicol. 2015, 39, 156–159. [Google Scholar] [CrossRef]
- Krpo, M.; Luytkis, H.C.; Haneborg, A.M.; Hoiseth, G. A Fatal Blood Concentration of 5-APB. Forensic Sci. Int. 2018, 291, E1–E3. [Google Scholar] [CrossRef]
- Hofmann, V.; Sundermann, T.R.; Landmann, A.; Rechtsteiner, S.; Schmitt, G.; Bartel, M. Simultaneous Determination of 5-and 6-APB in Blood, Other Body Fluids, Hair and Various Tissues by HPLC-MS/MS. J. Anal. Toxicol. 2022, 46, 264–269. [Google Scholar] [CrossRef]
- Rickli, A.; Kopf, S.; Hoener, M.C.; Liechti, M.E. Pharmacological Profile of Novel Psychoactive Benzofurans. Br. J. Pharmacol. 2015, 172, 3412–3425. [Google Scholar] [CrossRef]
- Bravo, R.R.; Carmo, H.; Silva, J.P.; Valente, M.J.; Carvalho, F.; Bastos, M.D.; da Silva, D.D. Emerging Club Drugs: 5-(2-Aminopropyl)Benzofuran (5-APB) Is More Toxic Than Its Isomer 6-(2-Aminopropyl)Benzofuran (6-APB) in Hepatocyte Cellular Models. Arch. Toxicol. 2020, 94, 609–629. [Google Scholar] [CrossRef]
- Brandt, S.D.; Walters, H.M.; Partilla, J.S.; Blough, B.E.; Kavanagh, P.V.; Baumann, M.H. The Psychoactive Aminoalkylbenzofuran Derivatives, 5-APB and 6-APB, Mimic the Effects of 3,4-Methylenedioxyamphetamine (Mda) on Monoamine Transmission in Male Rats. Psychopharmacology 2020, 237, 3703–3714. [Google Scholar] [CrossRef]
- Welter, J.; Kavanagh, P.; Meyer, M.R.; Maurer, H.H. Benzofuran Analogues of Amphetamine and Methamphetamine: Studies on the Metabolism and Toxicological Analysis of 5-APB and 5-MAPB in Urine and Plasma Using GC-MS and Lc-(Hr)-Msn Techniques. Anal. Bioanal. Chem. 2015, 407, 1371–1388. [Google Scholar] [CrossRef]
- Welter-Luedeke, J.; Maurer, H.H. New Psychoactive Substances: Chemistry, Pharmacology, Metabolism, and Detectability of Amphetamine Derivatives with Modified Ring Systems. Ther. Drug Monit. 2016, 38, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Labutin, A.V.; Temerdashev, A.Z. Identification of (2-Aminopropyl)Benzofuran and Its Metabolites in Human Urine. J. Anal. Chem. 2017, 72, 770–776. [Google Scholar] [CrossRef]
- Capela, J.P.; Macedo, C.; Capela, J.P.; Branco, P.S.; Ferreira, L.M.; Lobo, A.M.; Fernanades, E.; Remião, F.; Bastos, M.L.; Dirnagl, U.; et al. Neurotoxicity Mechanisms of Ecstasy Metabolites. Toxicol. Lett. 2007, 172, S39–S40. [Google Scholar] [CrossRef]
- Dallinger, D.; Pinho, V.D.; Gutmann, B.; Kappe, C.O. Laboratory-Scale Membrane Reactor for the Generation of Anhydrous Diazomethane. J. Org. Chem. 2016, 81, 5814–5823. [Google Scholar] [CrossRef]
- Rieche, A.; Gross, H.; Hoft, E. Uber Alpha-Halogenather. 4. Synthesen Aromatischer Aldehyde Mit Dichlormethylalkylathern. Chem. Ber. 1960, 93, 88–94. [Google Scholar] [CrossRef]
- Macedo, C.; Branco, P.S.; Ferreira, L.M.; Lobo, A.M.; Capela, J.P.; Fernandes, E.; Bastos, M.D.; Carvalho, F. Synthesis and Cyclic Voltammetry Studies of 3,4-Methylenedioxymethamphetamine (MDMA) Human Metabolites. J. Health Sci. 2007, 53, 31–42. [Google Scholar] [CrossRef]
- Rossi, S.; Benaglia, M.; Porta, R.; Cotarca, L.; Maragni, P.; Verzini, M. A Stereoselective Catalytic Nitroaldol Reaction as the Key Step in a Strategy for the Synthesis of the Renin Inhibitor Aliskiren. Eur. J. Org. Chem. 2015, 2015, 2531–2537. [Google Scholar] [CrossRef]
- Orlandi, M.; Tosi, F.; Bonsignore, M.; Benaglia, M. Metal-Free Reduction of Aromatic and Aliphatic Nitro Compounds to Amines: A HSiCl3-Mediated Reaction of Wide General Applicability. Org. Lett. 2015, 17, 3941–3943. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.I.; Tatchell, A.R.; Furnis, B.S.; Hannaford, A.J.; Smith, P.W.G. Vogel’s Textbook of Practical Organic Chemistry; Pearson Education Limited: Essex, UK, 1989; p. 432. [Google Scholar]
- Li, Y.H.; Wang, Z.C.; Wu, X.F. Palladium-Catalyzed Carbonylative Direct Transformation of Benzyl Amines under Additive-Free Conditions. ACS Catal. 2018, 8, 738–741. [Google Scholar] [CrossRef]
- Detterbeck, R.; Hesse, M. An Improved and Versatile Method for the Rapid Synthesis of Aryidihydrobenzofuran Systems by a Boron Tribromide-Mediated Cyclization Reaction. Helv. Chim. Acta 2003, 86, 343–360. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.L.; Fino, I.; Ferreira, L.M.; Branco, P.S. Synthesis of 2-(5-(2-Aminopropyl)-2-hydroxyphenyl)acetic Acid, a Metabolite of the Drug 5-APB. Molbank 2023, 2023, M1629. https://doi.org/10.3390/M1629
Silva AL, Fino I, Ferreira LM, Branco PS. Synthesis of 2-(5-(2-Aminopropyl)-2-hydroxyphenyl)acetic Acid, a Metabolite of the Drug 5-APB. Molbank. 2023; 2023(2):M1629. https://doi.org/10.3390/M1629
Chicago/Turabian StyleSilva, André L., Inês Fino, Luísa M. Ferreira, and Paula S. Branco. 2023. "Synthesis of 2-(5-(2-Aminopropyl)-2-hydroxyphenyl)acetic Acid, a Metabolite of the Drug 5-APB" Molbank 2023, no. 2: M1629. https://doi.org/10.3390/M1629
APA StyleSilva, A. L., Fino, I., Ferreira, L. M., & Branco, P. S. (2023). Synthesis of 2-(5-(2-Aminopropyl)-2-hydroxyphenyl)acetic Acid, a Metabolite of the Drug 5-APB. Molbank, 2023(2), M1629. https://doi.org/10.3390/M1629






