Abstract
The 2-(2,7-bis(pyridin-3-ylethynyl)fluoren-9-ylidene)malononitrile (1) was synthesized by reaction of 2,7-bis(pyridin-3-ylethynyl)fluoren-9-one with malononitrile in DMSO solution. The structural characterization of 1 by SC-XRD analysis was accompanied by elemental analysis, FT-IR, NMR, and MS measurements.
1. Introduction
Fluorene and its derivatives are essential tools for the fabrication of functional materials, including organic photovoltaics [1] and electronic devices [2]. Purely organic [3] and hybrid metal–organic frameworks [4] with tunable absorption and emission properties have been prepared.
Fluoren-9-ylidene malononitrile derivatives are an interesting family of fluorene compounds as they are easily accessible from fluoren-9-one precursors. The electron-rich nature of fluorene is modified by the presence of the two strong electron-withdrawing cyano groups, which cause stabilization of the lowest unoccupied molecular orbital (LUMO) [2,5].
In order to design mixed Py/CN polytopic building blocks and to explore their potential use in supramolecular chemistry, additional moieties (such as pyridyl groups) can be appended to the fluoren-9-ylidene malononitrile core. In fact, pyridyl derivatives have proven to be extremely versatile in the field of supramolecular chemistry since they can be reacted with a large variety of complementary building blocks such as halogens [6,7], boron derivatives [8], and metal ions/complexes [9]. Herein, we report the synthesis and structural characterization of the novel pyridyl-functionalized fluoren-9-ylidene malononitrile derivative 2-(2,7-bis(pyridin-3-ylethynyl)fluoren-9-ylidene)malononitrile (1) (Scheme 1).
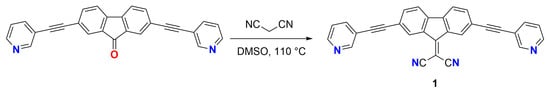
Scheme 1.
Synthesis of 2-(2,7-bis(pyridin-3-ylethynyl)fluoren-9-ylidene)malononitrile (1).
2. Results
Compound 2,7-bis(pyridin-3-ylethynyl)fluoren-9-one was prepared by Sonogashira coupling reaction [10] and then reacted with malononitrile in DMSO (Scheme 1) to give compound 1.
The multitopic ligand 1 was fully characterized by means of elemental analysis, ESI-MS, FT-IR, 1H, and 13C NMR spectroscopy. The middle infrared spectrum shows the characteristic peaks for the C≡N and C≡C stretching modes at 2225 and 2198 cm−1, respectively (Figure S1). The 1H NMR spectrum in CDCl3 (Figure S2) shows the H-pyridyl signals at 8.80, 8.59, 7.87, and 7.33 ppm, and those of the relevant protons of the fluoren-9-ylidene fragment at 8.55, 7.71, and 7.60 ppm. The 13C{1H} spectrum in the same solvent (Figure S3) shows a signal at 159.9 ppm assigned to the C−9 of the fluoren-9-ylidene moiety and three signals resonating at 91.5, 88.6, and 77.9 ppm ascribed to the alkyne and nitrile functionalities, respectively. The MS spectrum in MeCN is consistent with the chemical structure of compound 1 (Figure S4). Recrystallization of the crude product from chloroform yielded well-defined purple needle-shaped crystals, which were structurally characterized by SC-XRD analysis. Compound 1 crystallizes in the monoclinic space group P21/c with one molecule in the asymmetric unit featuring a periplanar conformation where the nitrogen atoms of both pyridyl rings face the same side of the malononitrile substituent (Figure 1) [7].
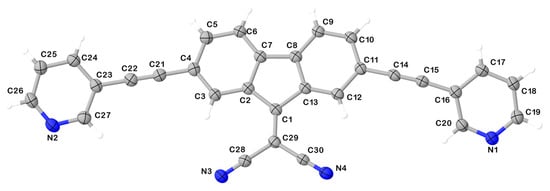
Figure 1.
Ellipsoid plot of compound 1 with the numbering scheme adopted. Displacement ellipsoids are drawn at 50 % probability level.
Crystal data for compound 1: C30H14N4, (Mr = 430.45 g mol−1) monoclinic, P21/c (No. 14), a = 7.0164(2) Å, b = 28.2624(6) Å, c = 11.2725(3) Å, β = 107.350(3) °, α = γ = 90 °, V = 2133.63(10) Å3, T = 100(2) K, Z = 4, Z’ = 1, µ(Cu Kα) = 0.637 mm−1, 17,137 reflections measured, 3,904 unique (Rint = 0.0573) which were used in all calculations. The final wR2 was 0.1540 (all data), and R1 was 0.0538 (I ≥ 2 σ(I)).
Compound 1 adopts a quasi-planar geometry with pyridyl rings twisted by about 7° and 8° with respect to the fluorenylidene moiety, resulting in an overall slightly wavy shape. The central bis(fluoren-9-ylidene)malononitrile core is involved in two intramolecular CH∙∙∙N hydrogen bonds between fluorenyl protons and nitrile functionalities identified as interactions a and b in Figure 2b and Table 1. A Full Interaction Map [11] (FIM) was calculated for compound 1 (based on IsoStar data from the CSD) to gain a better insight into the interaction preferences for the title compound (Figure 2a).
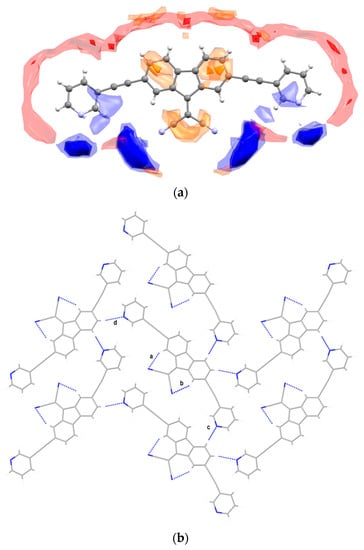
Figure 2.
(a) Full interaction map calculated for compound 1 showing regions of interaction’s likelihood for acceptors (red), donors (blue), and hydrophobic groups (orange). (b) Partial view of the crystal packing for compound 1 along the [1 0 0] direction showing intra- and intermolecular interactions labelled according to Table 1.

Table 1.
Intra- and intermolecular interactions of compound 1.
The FIM indicates four main hydrogen bond acceptor sites (highlighted in blue) close to the heteroatoms and a diffuse hydrogen bond donor region (highlighted in red) with hotspots close to the aryl protons of both the pyridyl and fluorenyl moieties. In agreement with the knowledge-based predicted scenario, the crystal packing is characterized by multiple intermolecular CH∙∙∙N bonds between adjacent molecules of compound 1, generating wavy sheets parallel to the [0 1 0] direction (Figure 3; see interactions c–d in Figure 2b and Table 1).
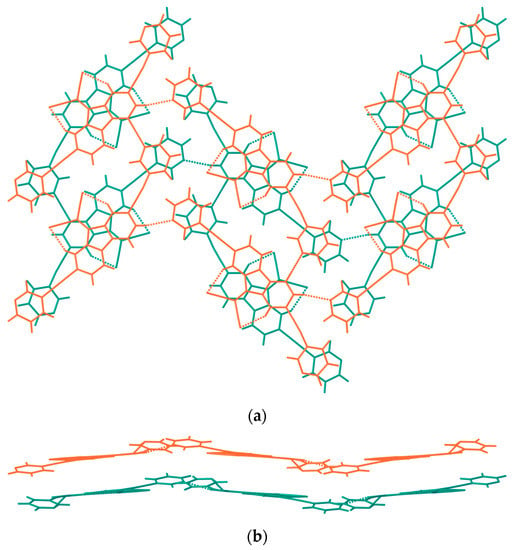
Figure 3.
Packing diagrams of compound 1 viewed along [1 0 0] and [1 0 1] directions in (a,b), respectively, showing corrugated sheets. Molecules from adjacent sheets are depicted in different colors. H-bonds are represented as dashed lines.
3. Materials and Methods
3.1. General
Solvents and reagents were purchased from TCI (Dublin, Ireland), FluoroChem (Dublin, Ireland), and Aldrich (Schnelldorf, Germany). Compound 2,7-bis(pyridin-3-ylethynyl)fluoren-9-one was prepared as previously described [10]. FT-IR spectroscopy measurements were recorded at room temperature on a Thermo-Nicolet 5700 (Burladingen, Germany) spectrometer using KBr pellets with a KBr beam splitter and KBr windows (4000–400 cm−1, resolution 4 cm−1). 1H and 13C NMR spectra were carried out in CDCl3 at room temperature using a Bruker Avance III HD 600 (Mannheim, Germany) spectrometer. Chemical shifts are reported in ppm (δ) and were calibrated to the solvent residue. Coupling constants J are expressed in Hertz (Hz). Positive ESI-MS spectra were recorded on a high-resolution LTQ Orbitrap Elite™ mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Solutions were infused into the ESI source at a flow rate of 5.00 μL/min. Spectra were recorded in the range of m/z 300–600 with a resolution of 240,000 (FWHM). Instrument conditions were as follows. Spray voltage 3,500 V, capillary temperature 275 °C, sheath gas 12 (arbitrary units), auxiliary gas 3 (arbitrary units), sweep gas 0 (arbitrary units), and probe heater temperature 50 °C. Elemental analysis was carried out with a CHNS/O PE 2400 series II elemental analyzer (Thermo Fisher, Dreieich, Germany; T = 925 °C). The melting point was determined on a FALC mod. C apparatus. X-ray diffraction data for compound 1 were collected at 100(2) K on a Rigaku FRE+ diffractometer equipped with VHF Varimax confocal mirrors, an AFC12 goniometer, and an HyPix 6000 detector. The structure was solved with the ShelXT [12] solution program using dual methods, and the model was refined with ShelXL 2018/3 [13] using full matrix least squares minimization on F2. Olex2 1.5 [14] was used as the graphical interface.
3.2. Synthesis of 2-(2,7-Bis(pyridin-3-ylethynyl)fluoren-9-ylidene)malononitrile (1)
Compound 1 was synthesized by a slight modification of a previously reported synthetic procedure [15]. 2,7-Bis(pyridin-3-ylethynyl)fluoren-9-one (38.2 mg; 0.100 mmol) and malononitrile (7.2 mg; 0.11 mmol) were reacted in DMSO (0.5 mL) at 110 °C for 5 h. The mixture was cooled to room temperature, and the dark solid was filtered on a Gooch funnel. The solid was then washed thoroughly with acetonitrile and dried under vacuum. Purple needle-shaped crystals suitable for SC-XRD analysis were grown by slow evaporation of a chloroform solution of the product (28.1 mg; 0.065 mmol; Y = 65 %) M. p. = 301 °C. ESI(+)-MS (MeCN solution) m/z 431.1322 for [C30H15N4]+ [M+H]+. Elemental analysis calcd (%) for C30H14N4: C 83.71, H 3.28, N 13.02. Found: C 83.27, H 2.79, N 13.88. FT−IR (KBr, 4000–400 cm−1): 3055 w, 3028 w, 2987 w, 2225 m ν(C≡N), 2198 w ν(C≡C), 1963 w, 1930 w, 1894 w, 1861 w, 1604 w, 1562 s, 1481 s, 1465 ms, 1427 m, 1408 ms, 1385 w, 1321 w, 1261 w, 1238 w, 1222 w, 1190 mw, 1176 mw, 1118 m, 1095 mw, 1024 ms, 987 w, 949 w, 922 w, 899 ms, 872 ms, 845 ms, 802 s, 756 ms, 700 s, 638 w, 615 w, 597 m, 551 w, 530 w, 513 w, 486 w, 438 w, 405 mw cm−1. 1H NMR (600 MHz, CDCl3) δ: 8.80 (s, 2H, Py), 8.59 (s, 2H, Py), 8.55 (s, 2H, fl), 7.87 (d, J = 6.6 Hz, 2H, Py), 7.71 (d, J = 7.0 Hz, 2H, fl), 7.60 (d, J = 7.0 Hz, 2H, fl), 7.33 (s, 2H, Py) ppm. 13C{1H} NMR (151 MHz, CDCl3) δ: 159.9, 152.3, 149.1, 141.6, 139.0, 138.1, 134.7, 129.9, 124.3, 123.3, 121.2, 120.0, 113.0, 91.5, 88.7, 77.9 ppm.
4. Conclusions
The compound 2-(2,7-bis(pyridin-3-ylethynyl)fluoren-9-ylidene)malononitrile (1) has been synthesized and fully characterized. Further studies are ongoing in our laboratories to evaluate the potential use of compound 1 as a building block for the formation of supramolecular assemblies.
Supplementary Materials
The following supporting information is available online; Figure S1: FT-IR; Figures S2 and S3: 1 H and 13 C{1 H} NMR; Figure S4: ESI(+) MS; Figure S5: π−π stacking interactions; Table S1: Crystal data and refinement parameters; Tables S2 and S3: Bond lengths and angles.
Author Contributions
Conceptualisation, M.C.A., E.P.; data curation, M.C.A., E.P., A.P., M.A., J.B.O. and S.J.C.; investigation, M.C.A., E.P., F.M., M.A., V.L., G.F., S.J.C., J.B.O. and A.P; writing (original draft), M.C.A. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge Fondazione di Sardegna (FdS Progetti Biennali di Ateneo, annualità 2018) for financial support and the EPSRC (Engineering and Physical Science Research Council) for the continued support of the UK’s National Crystallography Service (NCS), based at the University of Southampton.
Data Availability Statement
Crystallographic data were deposited at CCDC (CIF deposition number 2238742).
Acknowledgments
CeSAR (Centro Servizi di Ateneo per la Ricerca) of the University of Cagliari is kindly acknowledged for NMR and MS facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karak, S.; Homnick, P.J.; Renna, L.A.; Venkataraman, D.; Mague, J.T.; Lahti, P.M. Solution-Processed Photovoltaics with a 3,6-Bis(Diarylamino)Fluoren-9-Ylidene Malononitrile. ACS Appl. Mater. Interfaces 2014, 6, 16476–16480. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.A.; Neckers, D.C. Synthesis and Photophysics of Ambipolar Fluoren-9-Ylidene Malononitrile Derivatives. J. Org. Chem. 2009, 74, 8484–8487. [Google Scholar] [CrossRef] [PubMed]
- Baheti, A.; Justin Thomas, K.R.; Lee, C.P.; Li, C.T.; Ho, K.C. Organic Dyes Containing Fluoren-9-Ylidene Chromophores for Efficient Dye-Sensitized Solar Cells. J. Mater. Chem. A Mater. 2014, 2, 5766–5779. [Google Scholar] [CrossRef]
- Podda, E.; Arca, M.; Pintus, A.; Lippolis, V.; Caltagirone, C.; Coles, S.J.; Orton, J.B.; Ennas, G.; Picci, G.; Davies, R.P.; et al. On the role of torsional dynamics in the solid-state fluorescent properties of a new bifluorene-tetracarboxylic acid and its supramolecular assemblies: A structural and TD-DFT investigation. CrystEngComm 2023, 25, 1058–1066. [Google Scholar] [CrossRef]
- Homnick, P.J.; Tinkham, J.S.; Devaughn, R.; Lahti, P.M. Engineering Frontier Energy Levels in Donor-Acceptor Fluoren-9-Ylidene Malononitriles versus Fluorenones. J. Phys. Chem. A 2014, 118, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Aragoni, M.C.; Podda, E.; Arca, M.; Pintus, A.; Lippolis, V.; Caltagirone, C.; Bartz, R.H.; Lenardão, E.J.; Perin, G.; Schumacher, R.F.; et al. An Unprecedented Non-Classical Polyinterhalogen Anion Made of [I2Cl]− and I2 at the 2-(p-Tolyl)Selenopheno[2,3-b]Pyridinium Cation Template. New J. Chem. 2022, 46, 21921–21929. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Caltagirone, C.; Castellano, C.; Demartin, F.; Garau, A.; Isaia, F.; Lippolis, V.; Montis, R.; Pintus, A. Cationic and Anionic 1D Chains Based on NH+⋯N Charge-Assisted Hydrogen Bonds in Bipyridyl Derivatives and Polyiodides. CrystEngComm 2012, 14, 5809–5823. [Google Scholar] [CrossRef]
- Podda, E.; Coles, S.J.; Horton, P.N.; Lickiss, P.D.; Bull, O.S.; Orton, J.B.; Pintus, A.; Pugh, D.; Carla Aragoni, M.; Davies, R.P. First Example of Solid-State Luminescent Borasiloxane-Based Chiral Helices Assembled through N–B Bonds. Dalton Trans. 2021, 50, 3782–3785. [Google Scholar] [CrossRef] [PubMed]
- Podda, E.; Arca, M.; Coles, S.J.; Crespo Alonso, M.; Isaia, F.; Pintus, A.; Lippolis, V.; Aragoni, M.C. Supramolecular Assemblies Tailored by Dipyridyl-1,2-4-Thiadiazoles: Influence of the Building Blocks in the Predictability of the Final Network. Supramol. Chem. 2020, 32, 267–275. [Google Scholar] [CrossRef]
- Podda, E.; Arca, M.; Pintus, A.; Demontis, V.; Lippolis, V.; Ferino, G.; Orton, J.B.; Coles, S.J.; Aragoni, M.C. 2,7-bis(pyridin-3-ylethynyl)fluoren-9-one. Molbank 2023, 2023, M1540. [Google Scholar] [CrossRef]
- Wood, P.A.; Olsson, T.S.G.; Cole, J.C.; Cottrell, S.J.; Feeder, N.; Galek, P.T.A.; Groom, C.R.; Pidcock, E. Evaluation of Molecular Crystal Structures Using Full Interaction Maps. CrystEngComm 2012, 15, 65–72. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Wong, W.Y.; Lu, G.L.; Choi, K.H.; Lin, Z. Functionalization of 9-(Dicyanomethylene)Fluorene Derivatives with Substituted Acetylenes. Eur. J. Org. Chem. 2003, 2003, 365–373. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).