Abstract
In this paper, a synthesis and characterization of a novel three coordinate 2-{9-(10-Bromoanthracenyl)}-1,3-dihydro-1H-[d]-1,3,2-benzodiazaborole from a cyclo-condensation reaction of o-phenylenediamine and 10-bromoanthracene-9-boronic acid is described. The desired product was obtained in 0.343 g (92% yields) with its structure characterized by 1H, 11B, 13C NMR, HRMS and FT-IR.
1. Introduction
The development of novel three and four-coordinate organoboron compounds with rigid structures have attracted significant research interest because of their wide range of applications in biological imagining as well as in organic electronics [1,2,3,4,5,6]. These compounds enjoy excellent photophysical and electronic properties due to electronic communication between the electron-deficient boron atom and an adjacent electron-rich π-conjugated system, and have been used as emitters in organic light emitting diodes (OLEDs) [7], as fluorogenic and ratiometric chemo-sensors for fluoride anions [8,9,10]. Amongst these, organoboron fluorophores chelated with N,N-, N,O- and N,C-chromophores as ligands, have been shown to possess exceptional spectroscopic properties such as strong absorption and emission bands which can easily be fine-tuned through simple structural changes [11,12,13]. More especially, boron dipyrromethanes (BODIPYs) have been the center of the largest number of investigations in this realm due to their excellent structural versatility, thermalization, and photostability [14,15,16,17,18,19]. Even though BODIPYs are strongly emitters in solution, their solid states are almost non-emissive, owing to a self-absorption phenomenon caused by small stroke shifts and an intermolecular π-π stacking interaction enhanced by structural planarity [20]. To overcome molecular planarity, Gai and coworkers recently reported a series of five- and six-membered-ring bis- (diphenylboron) complexes in which the fluorine atoms are replaced with bulky phenyl rings [21]. Although the resulting dyes showed improved photophysical properties in both solution and in solid state, the overlapping π-π interaction between the fluorophores, which in turn resulted in relative low fluorescence in solid state, is still an obstacle [22]. Therefore, we considered that designing three-coordinate boron compounds with a larger anthracene group, directly bonded to the boron atom, might block π-π interaction between organoboron fluorophores.
2. Results and Discussion
The cyclocondensation of 1,8-diaminonaphthalene or o-phenylene diamines with different aromatic boronic acids, resulted in an efficient synthesis of the desired benzodiazaborole and naphthodiazaborinane derivatives [23]. A series of desired organoboron compounds were successfully synthesized affording some novel compounds, 2-{9-(10-bromoanthracenyl)}-1,3-dihydro-1H-[d]-1,3,2-benzodiazaborole being one of them.
The formation of the synthesized compound was characterized using 1HNMR, 11B NMR, 13CNMR spectroscopy, FT-IR and high-resolution mass spectroscopy. 1HNMR spectrum showed characteristic broad singlet peak for NH groups resonating at 6.95 ppm and distinct ortho and para-positioned pairs of protons each integrating for two on the benzo ring (Figure S1). Two doublet peaks, each integrating for two, are assigned to a pair of anthracenyl protons due to their structural plane of symmetry. The 13CNMR spectrum (Figure S2) further confirmed the formation of the desired diazaborole showing aromatic peaks in the range of 111–136.10 ppm. In the FT-IR of the compound (Figure S3), the sharp absorption bands at 3450.07 and 3401.45 cm−1 assigned to the NH groups confirmed a successful fived-membered ring closure that are due to an unsymmetrical NH bond stretching. The intense peak at 741.94 cm−1 can be assigned to the C-Br vibration signal. The structure was additionally confirmed with mass spectrum (Figure S4) showing a molecular ion m/z 373.0515. The presence of Br was established due to the presence of the M+2 peak at m/z 375.0433 because of 81Br isotope. The singlet peak on the 11B NMR spectrum (Figure S5), resonating at 29.64 ppm, reinforced the presence of the boron atom.
3. Materials and Methods
All chemicals were purchased from commercial suppliers and used without purification. 10-Bromoanthracene-9-boronic acid (98%) and o-phenylenediamine were purchased from Sigma-Aldrich Corporation (St. Louis, MI, USA). All solvents were purchased from Sigma-Aldrich and were of analytical grade. 1HNMR, 13CNMR and 11B NMR spectra were acquired on Bruker advance III 400 MHz NMR spectroscopy with a 5 mm TBIZ probe at 25 °C. Chemical shifts were reported in ppm in relation to the solvent (DCCl3) residual peak, at 7.26 ppm for 1HNMR and 77.16 ppm for 13CNMR. Coupling constants (J) were calculated in hertz (Hz). The infrared spectrum was recorded using a Bruker Alpha II FT-IR spectrometer and the data were reported as a percentage transmittance at the respective wavenumbers (cm−1). The HRMS were obtained from high-resolution mass spectra (HRMS), obtained on a Waters Acquits LCT premier (TOF) ultra-performance liquid chromatography–mass spectrometry. Melting points were measured on a Reichert Austria apparatus using 22 × 22 mm deck Glaser. Exemplary 1H NMR, 13CNMR, 11B NMR, IR spectra and HRMS of the titled compound shown in Figures S1–S5, supplementary materials.
Synthesis of 2-{9-(10-Bromoanthracenyl)}-1,3-dihydro-1H-[d]-1,3,2-benzodiazaborole
The novel compound, 2-{9-(10-bromoanthracenyl)}-1,3-dihydro-1H-[d]-1,3,2-benzodiazaborole synthesized from the cyclo-condensation reaction of o-phenylene diamine and 10-bromoanthracene-9-boronic acid in refluxing toluene in the presence of anhydrous magnesium sulphate (Scheme 1). A 100 mL round bottomed-flask equipped with a magnetic stirrer bar was charged with 10-bromoanthracene-9-boronic acids (300.94 mg, 1.0 mmol), o-phenylene diamine (108.1 mg, 1.0 mmol), magnesium sulphate (692 mg, 5.0 mmol) and toluene (5.0 mL). The flask was fitted with a reflux condenser and the content heated to efflux for 3 hours. After heating, the reaction was allowed to cool to room temperature and the solvent removed under vacuum. The resulting residue was dissolved in minimal dichloromethane followed by purification using column chromatography. The titled compound was obtained as yellow crystalline product (0.343 g, 92%) with mp. 236–237 °C. 1HNMR (CDCl3, 400 Hz, δ ppm): 6.95 (br. s, 2 × NH), 7.10–7.15 (m, 2H, Ha), 7.25–7.30 (m, 2H), 7.41–7.47 (m, 2 H), 7.59–7.65 (m, 2 H), 8.10 (d, J = 8.68 Hz, 2 H), 8.63 (d, J = 8.87 Hz, 2 H). 11B NMR (CDCl3, 128 MHz, δ ppm): 29.64 (br. s). 13 C NMR (CDCl3, 100 Hz, δ ppm): 111.41, 119.75, 124.64, 125.45, 126.92128.17, 129.82, 130.15, 136.04, 136.12. FT-IR (cm−1): 3450, 3401, 1579, 1438, 1293, 1165, 741. HRMS: calculated for C20H15N2BrB m/z 373.0515, found [M+] 373.0512.
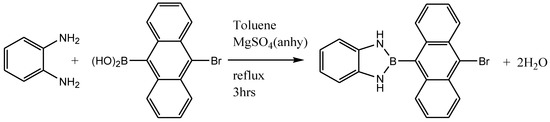
Scheme 1.
Synthetic path for 2-{9-(10-bromoanthracenyl)}-1,3-dihydro-1H-[d]-1,3,2-benzodiazaborole.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1: 1HNMR spectrum of 2-{9-(10-Bromoanthracenyl)}-1,3-dihydro-1H-[d]-1,3,2-benzodiazaborole; Figure S2: 13 C NMR of 2-{9-(10-Bromoanthracenyl)}-1,3-dihydro-1H-[d]-1,3,2-benzodiazaborole; Figure S3: FT-IR of 2-{9-(10-Bromoanthracenyl)}-1,3-dihydro-1H-[d]-1,3,2-benzodiazaborole; Figure S4: HRMS of 2-{9-(10-Bromoanthracenyl)}-1,3-dihydro-1H-[d]-1,3,2-benzodiazaborole; Figure S5: 11B NMR of 2-{9-(10-Bromoanthracenyl)}-1,3-dihydro-1H-[d]-1,3,2-benzodiazaborole.
Funding
This research was funded by National Research Foundation of South Africa and the University of KwaZulu Natal (no grant number).
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank the University of KwaZulu natal for laboratory space to conduct research.
Conflicts of Interest
The author declares no conflict of interest.
References
- Entwistle, C.D.; Marder, T.B. Applications of Three-Coordinate Organoboron Compounds and Polymers in Optoelectronics. Chem. Mater. 2004, 16, 4574–4585. [Google Scholar] [CrossRef]
- Entwistle, C.D.; Marder, T.B. Boron Chemistry Lights the Way: Optical Properties of Molecular and Polymeric Systems. Angew. Chem. Int. Ed. 2002, 41, 2927–2931. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.; Popovic, Z.D.; Hu, N.X.; Hor, A.M.; Xu, G. Degradation Mechanism of Small Molecule-Based Organic Light-Emitting Devices. Science 1999, 283, 1900–1902. [Google Scholar] [CrossRef]
- Fischer, G.M.; Jüngst, C.; Isomäki-Krondahl, M.; Gauss, D.; Mӧller, H.M.; Daltrozzo, E.; Zumbusch, A. Asymmetric PPCys: Strongly fluorescing NIR labels. Chem. Commun. 2010, 46, 5289–5291. [Google Scholar] [CrossRef]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.D.; Bradley, D.C.; Dos Santos, D.A.; Brédas, J.; Lögdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Jia, W.-L.; Bai, D.-R.; McCormick, T.; Liu, Q.-D.; Motala, M.; Wang, R.Y.; Seward, C.; Tao, Y.; Wang, S. Three-Coordinate Organoboron Compounds BAr2R (Ar = Mesityl, R = 7-Azaindolyl- or 2,2′-Dipyridylamino-Functionalized Aryl or Thienyl) for Electroluminescent Devices and Supramolecular Assembly. Chem. Eur. J. 2004, 10, 994–1006. [Google Scholar] [CrossRef]
- Kubo, Y.; Yamamoto, M.; Ikeda, M.; Takeuchi, M.; Shinkai, S.; Yamaguchi, S.; Tamao, K. A Colorimetric and Ratiometric Fluorescent Chemosensor with Three Emission Changes: Fluoride Ion Sensing by a Triarylborane–Porphyrin Conjugate. Angew. Chem. Int. Ed. 2003, 42, 2036–2040. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Akiyama, S.; Tamao, K. Colorimetric Fluoride Ion Sensing by Boron-Containing π-Electron Systems. J. Am. Chem. Soc. 2001, 123, 11372–11375. [Google Scholar] [CrossRef]
- Parab, K.; Venkatasubbaiah, K.; Jäkle, F. Luminescent Triarylborane-Functionalized Polystyrene: Synthesis, Photophysical Characterization, and Anion-Binding Studies. J. Am. Chem. Soc. 2006, 128, 12879–12885. [Google Scholar] [CrossRef]
- Tamgho, I.-S.; Hasheminasab, A.; Engle, J.T.; Nemykin, V.N.; Ziegler, C.J. A New Highly Fluorescent and Symmetric Pyrrole–BF2 Chromophore: BOPHY. J. Am. Chem. Soc. 2014, 136, 5623–5626. [Google Scholar] [CrossRef] [PubMed]
- Frath, D.; Massue, J.; Ulrich, G.; Ziessel, R. Luminescent Materials: Locking π-Conjugated and Heterocyclic Ligands with Boron(III). Angew. Chem. Int. Ed. 2014, 53, 2290–2310. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, Q.-D.; Bai, D.-R.; Jia, W.-L.; Tao, Y.; Wang, S. Organoboron Compounds with an 8-Hydroxyquinolato Chelate and Its Derivatives: Substituent Effects on Structures and Luminescence. Inorg. Chem. 2005, 44, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef]
- Lu , H.; Gai, Q.L.; Li, Z.; Deng, Y.; Xiao, X.; Lai, G.; Shen, Z. Tuning the Solid-State Luminescence of BODIPY Derivatives with Bulky Arylsilyl Groups: Synthesis and Spectroscopic Properties. Chem.—Eur. J. 2012, 18, 7852–7861. [Google Scholar] [CrossRef]
- Gai, L.; Lu, H.; Zou, B.; Lai, G.; Shen, Z.; Li, Z. Synthesis, and spectroscopic properties of bodipy dimers with effective solid-state emission. RSC Adv. 2012, 2, 8840–8846. [Google Scholar] [CrossRef]
- Nepomnyashchii, A.B.; Pistner, A.J.; Bard, A.J.; Rosenthal, J. Synthesis, Photophysics, Electrochemistry and Electrogenerated Chemiluminescence of PEG-Modified BODIPY Dyes in Organic and Aqueous Solutions. J. Phys. Chem. C 2013, 117, 5599–5609. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, Y.; Xiao, Y.; Yu, G.; Liu, Y.; Qian, X. Bulky 4-tritylphenylethynyl substituted boradiazaindacene: Pure red emission, relatively large Stokes shift and inhibition of self-quenching. Chem. Commun. 2008, 39, 4777–4779. [Google Scholar] [CrossRef]
- Gai, L.; Xu, J.; Wu, Y.; Lu, H.; Shen, Z. Synthesis, and spectroscopic properties of novel N–N linked bis-(diphenylboron) complexes. New J. Chem. 2016, 40, 5752–5757. [Google Scholar] [CrossRef]
- Yu, C.; Jiao, L.; Zhang, P.; Feng, Z.; Cheng, C.; Wei, Y.; Mu, X.; Hao, E. Highly Fluorescent BF2 Complexes of Hydrazine–Schiff Base Linked Bispyrrole. Org. Lett. 2014, 16, 3048–3051. [Google Scholar] [CrossRef] [PubMed]
- Sithebe, S.; Robinson, R.S. Palladium-catalysed cross-coupling reaction of ultra-stabilised 2-aryl-1,3-dihydro-1H-benzo[d]1,3,2-diazaborole compounds with aryl bromides: A direct protocol for the preparation of unsymmetrical biaryls. Beilstein J. Org. Chem. 2014, 10, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).