N-isobutyl-1,8-bis(isobutylamino)-naphthalimide
Abstract
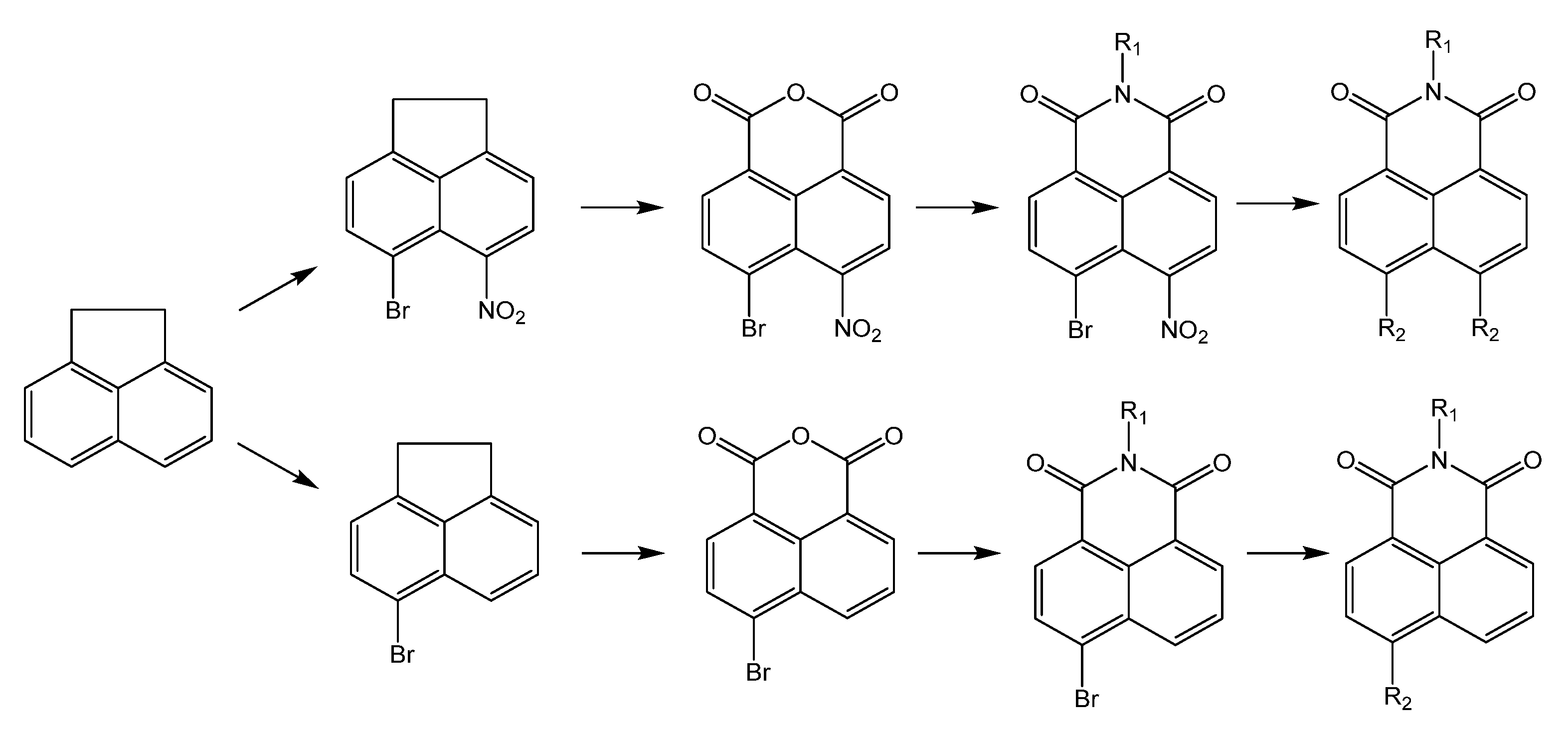
1. Introduction
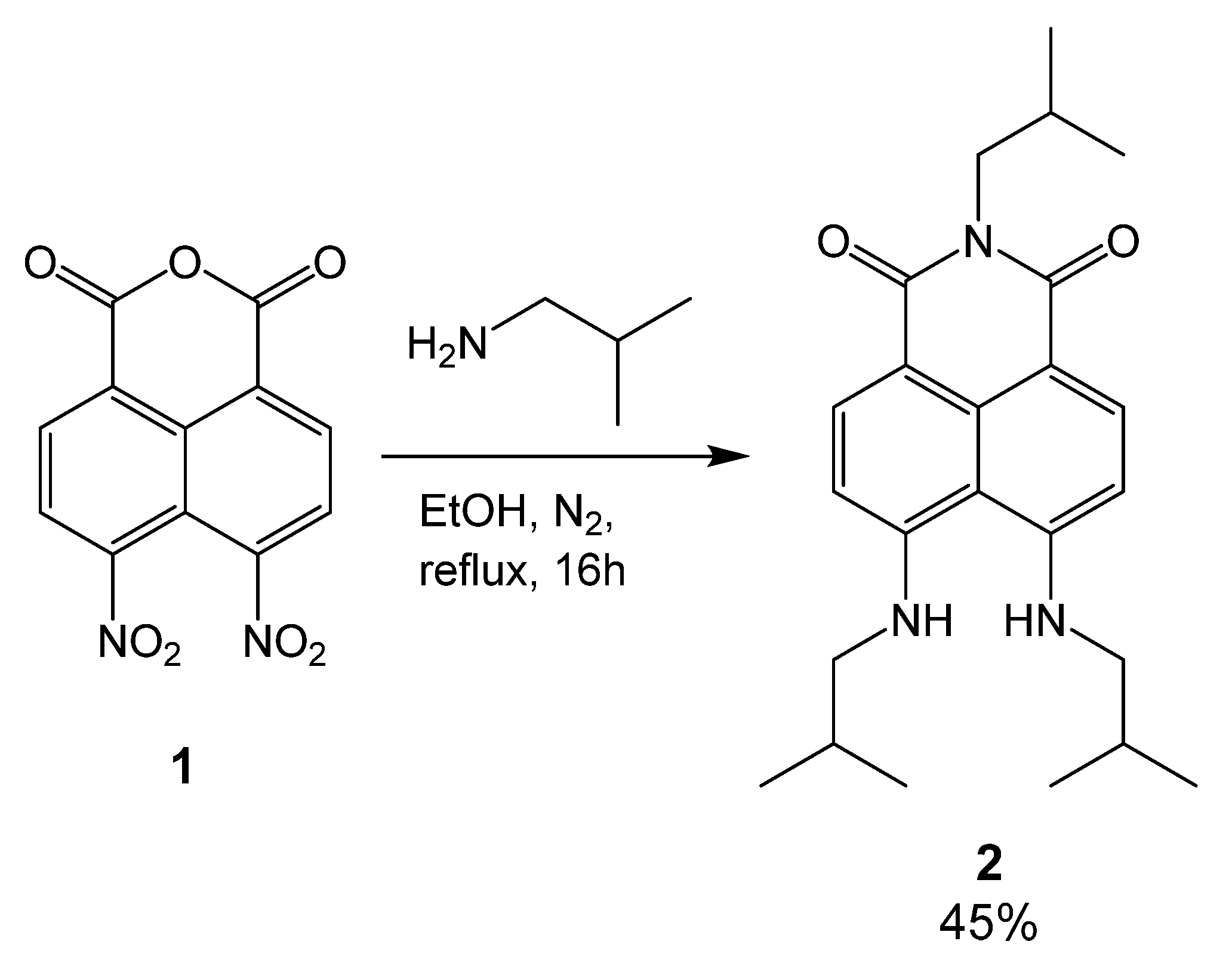
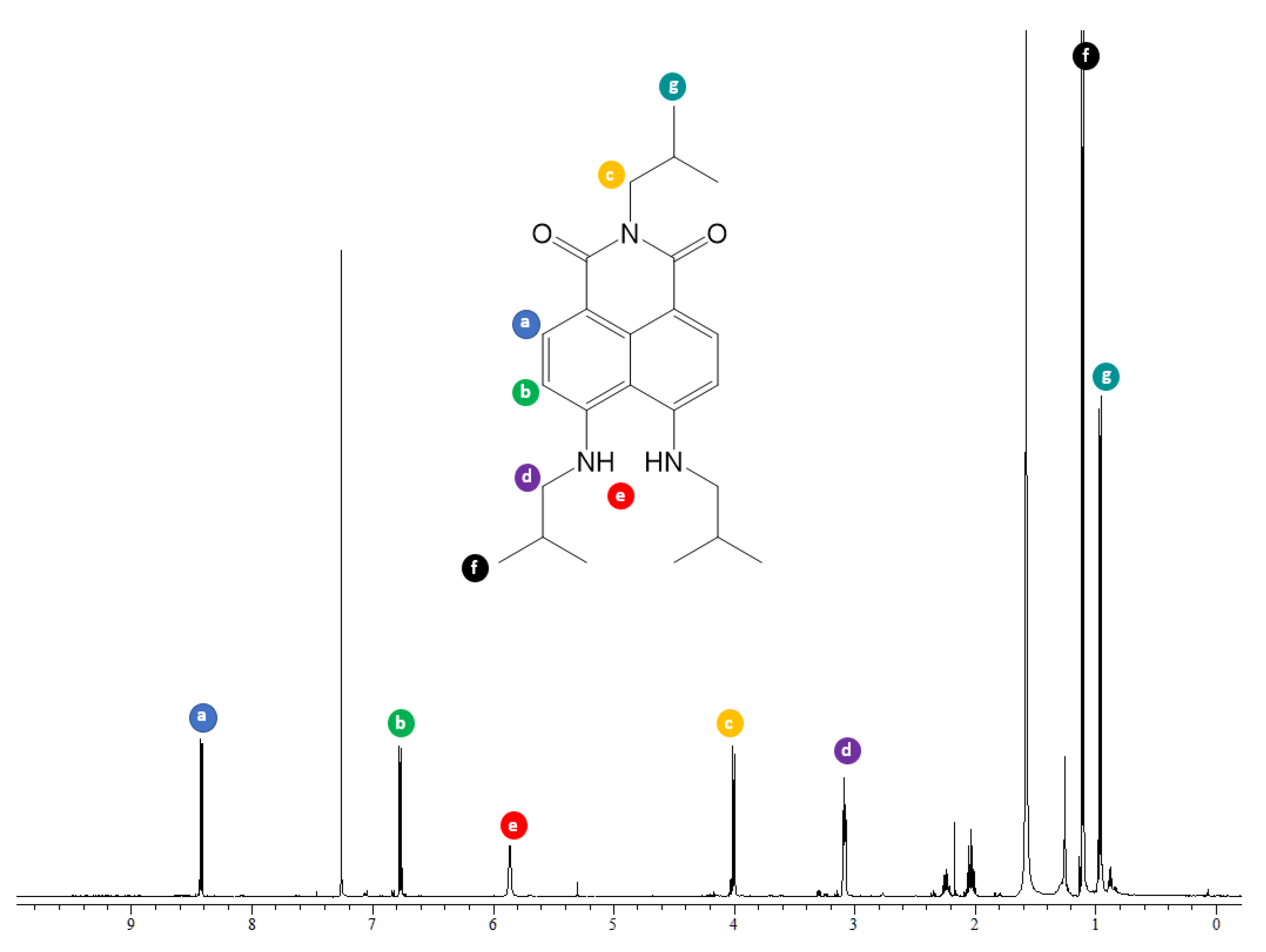
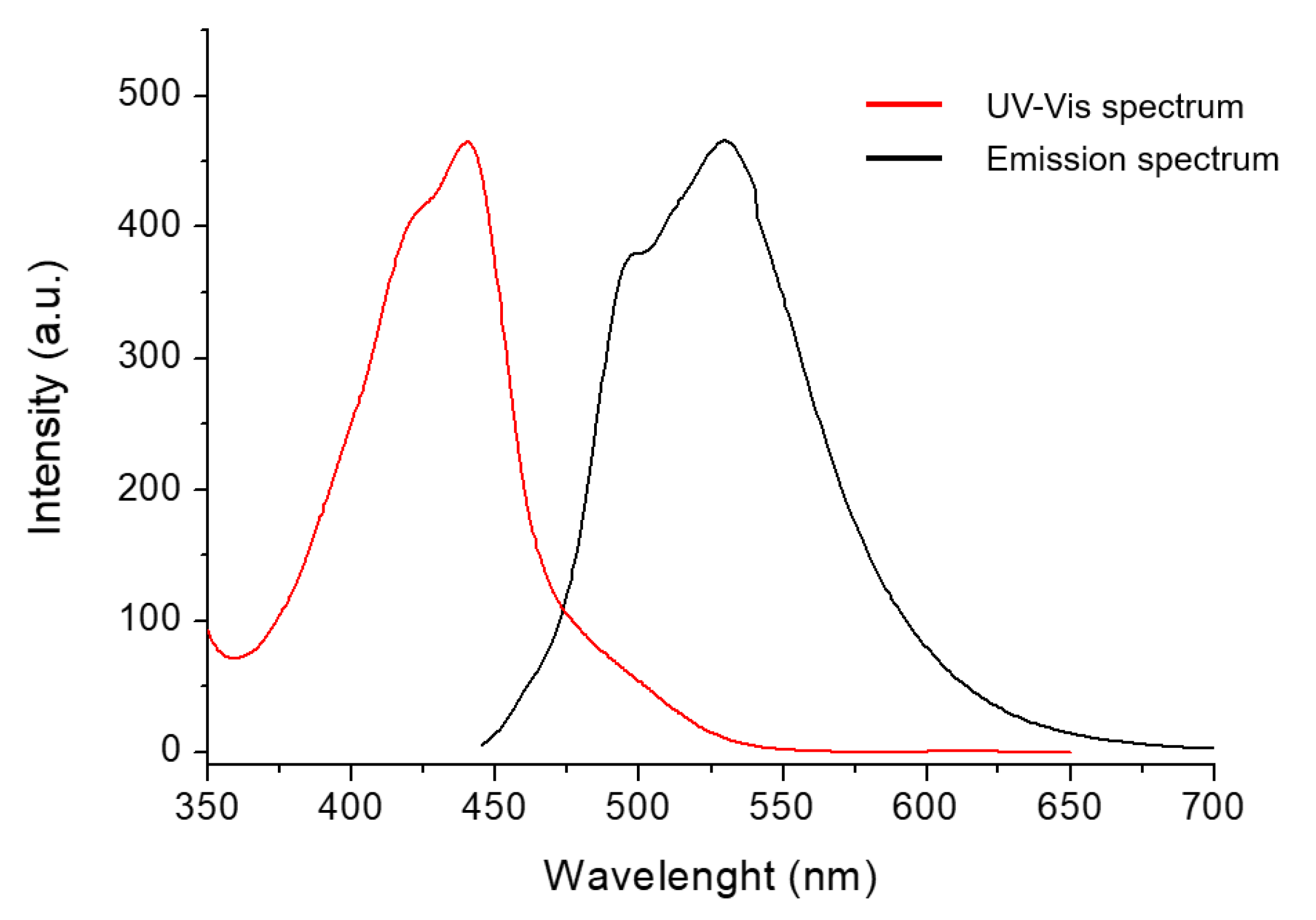
2. Results
3. Experimental Section
3.1. General
3.2. Synthesis of 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nisha, J.; Navneet, K. A comprehensive compendium of literature of 1,8-Naphthalimide based chemosensors from 2017 to 2021. Coord. Chem. Rev. 2022, 459, 214454–214497. [Google Scholar]
- Puglisi, R.; Pappalardo, A.; Gulino, A.; Trusso Sfrazzetto, G. Multitopic Supramolecular Detection of Chemical Warfare Agents by Fluorescent Sensors. ACS Omega 2019, 4, 7550–7555. [Google Scholar] [CrossRef]
- Tuccitto, N.; Spitaleri, L.; Li Destri, G.; Pappalardo, A.; Gulino, A.; Trusso Sfrazzetto, G. Supramolecular Sensing of a Chemical Warfare Agents Simulant by Functionalized Carbon Nanoparticles. Molecules 2020, 25, 5731. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef] [PubMed]
- Trusso Sfrazzetto, G.; Satriano, C.; Tomaselli, G.A.; Rizzarelli, E. Synthetic fluorescent probes to map metallostasis and intracellularfate of zinc and copper. Coord. Chem. Rev. 2016, 311, 125–167. [Google Scholar] [CrossRef]
- Satriano, C.; Trusso Sfrazzetto, G.; Amato, M.E.; Ballistreri, F.P.; Copani, A.; Giuffrida, M.L.; Grasso, G.; Pappalardo, A.; Rizzarelli, E.; Tomaselli, G.A.; et al. A ratiometric naphthalimide sensor for live cell imaging of copper(I). Chem. Commun. 2013, 49, 5565–5567. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.L.; Trusso Sfrazzetto, G.; Satriano, C.; Zimbone, S.; Tomaselli, G.A.; Copani, A.; Rizzarelli, E. A New Ratiometric Lysosomal Copper(II) Fluorescent Probe To Map a Dynamic Metallome in Live Cells. Inorg. Chem. 2018, 57, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Gopala, L.; Cha, Y.; Lee, M.H. Versatile naphthalimides: Their optical and biological behavior and applications from sensing to therapeutic purposes. Dye. Pigment. 2022, 201, 110195. [Google Scholar] [CrossRef]
- Rayappa Naveen, K.; Prabhu, C.P.K.; Braveenth, R.; Hyuk Kwon, J. Molecular Design Strategy for Orange Red Thermally Activated Delayed Fluorescence Emitters in Organic Light-Emitting Diodes (OLEDs). Chem. Eur. J. 2022, 28, e202103532. [Google Scholar] [PubMed]
- Santonocito, R.; Tuccitto, N.; Cantaro, V.; Carbonaro, A.B.; Pappalardo, A.; Greco, V.; Buccilli, V.; Maida, P.; Zavattaro, D.; Sfuncia, G.; et al. Smartphone-Assisted Sensing of Trinitrotoluene by Optical Array. ACS Omega 2022, 7, 37122–37132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, J.; Yu, G.; Chen, H.; Huang, J.; Liu, Y. Dialkyl-14H-benzo [4,5]isoquino [2,3-a]perimidin-14-one-3,4,10,11-tetracarboxylic diimides: A New Family of n-Type Organic Semiconductors. Chem. Asian J. 2012, 7, 2208–2212. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santonocito, R.; Caruso, I.M.; Trusso Sfrazzetto, G. N-isobutyl-1,8-bis(isobutylamino)-naphthalimide. Molbank 2023, 2023, M1568. https://doi.org/10.3390/M1568
Santonocito R, Caruso IM, Trusso Sfrazzetto G. N-isobutyl-1,8-bis(isobutylamino)-naphthalimide. Molbank. 2023; 2023(1):M1568. https://doi.org/10.3390/M1568
Chicago/Turabian StyleSantonocito, Rossella, Ivana Maria Caruso, and Giuseppe Trusso Sfrazzetto. 2023. "N-isobutyl-1,8-bis(isobutylamino)-naphthalimide" Molbank 2023, no. 1: M1568. https://doi.org/10.3390/M1568
APA StyleSantonocito, R., Caruso, I. M., & Trusso Sfrazzetto, G. (2023). N-isobutyl-1,8-bis(isobutylamino)-naphthalimide. Molbank, 2023(1), M1568. https://doi.org/10.3390/M1568







