Synthesis of Pentacycloundecane (PCUD) Based Spiro-Pyrano-Cage Framework via Ring-Closing Metathesis
Abstract
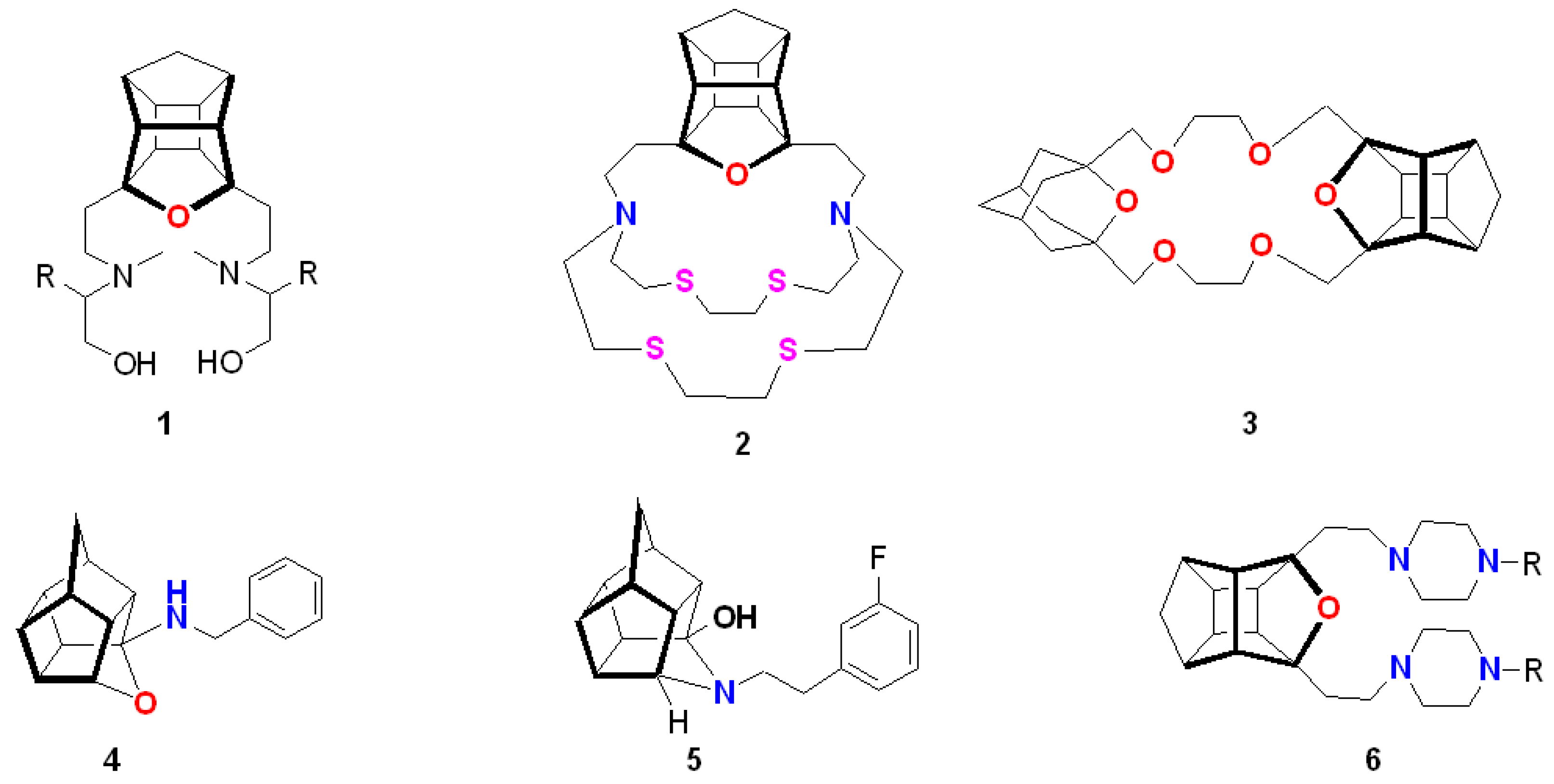
1. Introduction
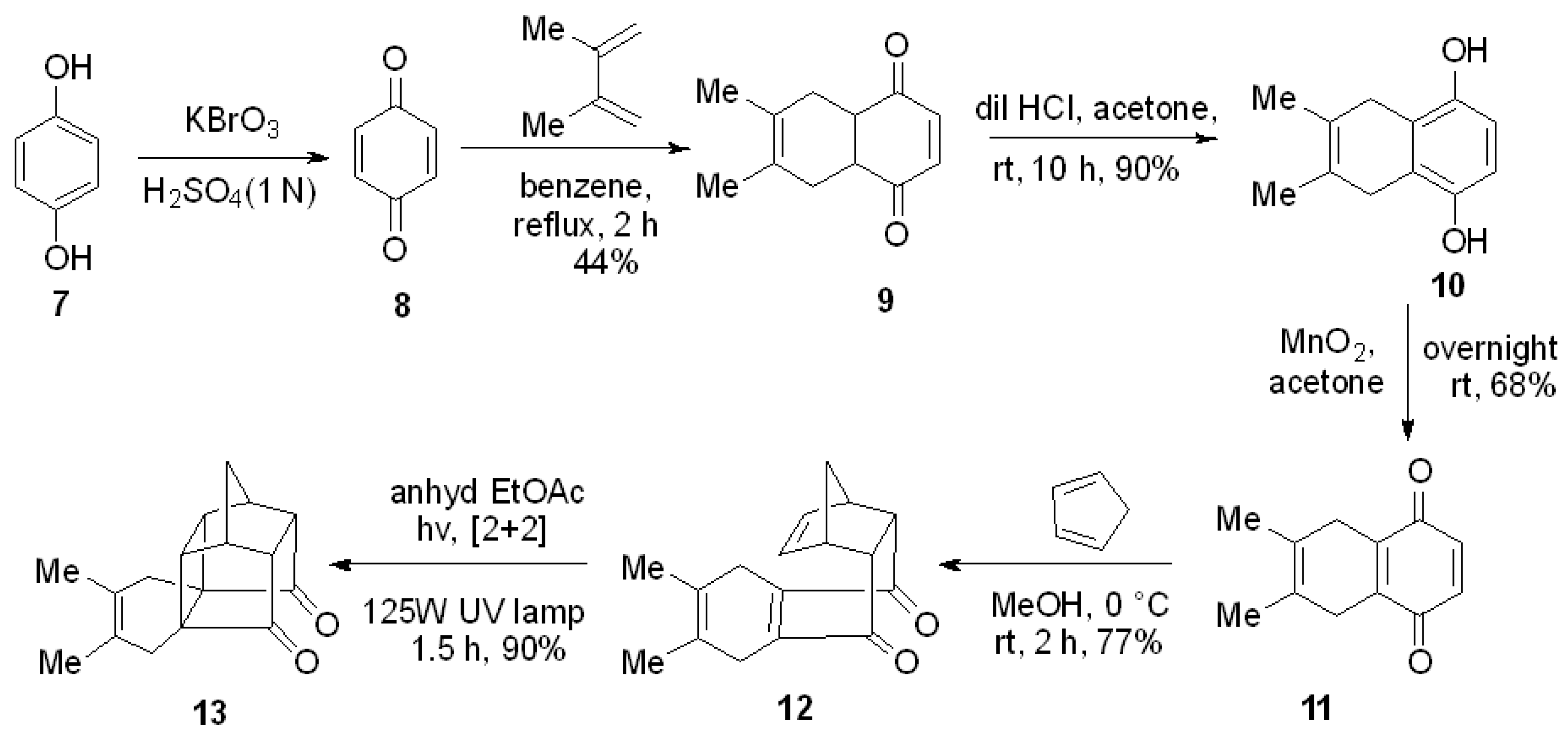
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization
3.2.1. Synthesis of Cage Diol 14 via Grignard Addition
3.2.2. Synthesis of Cage Triallyl Derivative 16
3.2.3. Synthesis of Compound Cage Spiro-Pyran 17 via RCM Protocol
3.2.4. Hydrogenation of Cage Spiro-Pyran 17
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomstyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar]
- Katritzky, A.R. Advances in Heterocyclic Chemistry; Academic Press: New York, NY, USA, 1963. [Google Scholar]
- Geldenhuys, W.J.; Malan, S.F.; Bloomquist, J.R.; Marchand, A.P.; Van der Schyf, C.J. Pharmacology, and structure-activity relationships of bioactive polycyclic cage compounds: A focus on pentacycloundecane derivatives. Med. Res. Rev. 2005, 25, 21–48. [Google Scholar] [CrossRef]
- Kotha, S.; Cheekatla, S.R.; Lal, S.; Mallick, L.; Kumbhakarna, N.; Chowdhury, A.; Namboothiri, I.N.N. Pentacycloundecane (PCUD)-Based Cage Frameworks as Potential Energetic Materials: Syntheses and Characterization. Asian J. Org. Chem. 2020, 9, 2116–2126. [Google Scholar] [CrossRef]
- Marchand, A.P.; McKim, S.A.; Kumar, K.A. Synthesis and alkali metal picrate extraction capabilities of novel, cage-functionalized diazacrown ethers. Effects of host preorganization on avidity and selectivity toward alkali metal picrates in solution. Tetrahedron 1998, 54, 13421. [Google Scholar] [CrossRef]
- Levitskaia, T.G.; Moyer, B.A.; Bonnesen, P.V.; Marchand, A.P.; Krishnudu, K.; Chen, Z.; Huang, Z.; Kruger, H.G.; Mckim, A.S. Novel Approach to Sodium Hydroxide Separation: Synergistic Pseudo-Hydroxide Extraction by a Fluorinated Alcohol and Cage-Functionalized Crown Ethers. J. Am. Chem. Soc. 2001, 123, 12099. [Google Scholar] [CrossRef]
- Lebedeva, M.A.; Chamberlain, T.W.; Khlobystov, A.N. Harnessing the Synergistic and Complementary Properties of Fullerene and Transition-Metal Compounds for Nanomaterial Application. Chem. Rev. 2015, 115, 11301. [Google Scholar] [CrossRef]
- Wilkinson, S.M.; Gunosewoyo, H.; Barron, M.L.; Boucher, A.; McDonnell, M.; Turner, P.; Morrison, D.E.; Bennett, M.R.; McGregor, I.S.; Rendina, L.M.; et al. The First CNS-Active Carborane: A Novel P2X7 Receptor Antagonist with Antidepressant Activity. ACS Chem. Neurosci. 2014, 5, 335–339. [Google Scholar] [CrossRef]
- Onajole, O.K.; Coovadia, Y.; Kruger, H.G.; Maguire Pillay, M.; Govender, T. Novel polycyclic ‘cage’-1,2-diamines as potential anti-tuberculosis agents. Eur. J. Med. Chem. 2012, 54, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boyle, G.A.; Govender, T.; Kruger, H.G.; Maguire, G.E.M. Synthesis of chiral pentacyclo-undecane ligands and their use in the enantioselective alkylation of benzaldehyde with diethylzinc. Tetrahedron Asymmetry 2004, 15, 2661. [Google Scholar] [CrossRef]
- Williams, S.M.; Brodbelt, J.S.; Marchand, A.P.; Cal, D.; Mlinarié-Majerski, K. Metal Complexation of Thiacrown Ether Macrocycles by Electrospray Ionization Mass Spectrometry. Anal. Chem. 2002, 74, 4423–4433. [Google Scholar] [CrossRef] [PubMed]
- Marchand, A.P.; Hazlewood, A.; Huang, Z.; Vadlakonda, S.K.; Rocha, J.-D.R.; Power, T.D.; Mlinaric-Majerski, K.; Klaic, L.; Kragol, G.; Bryan, J.C. Stabilization of a K+-(bis-Cage-Annulated 20-Crown-6) Complex by Bidentate Picrate. Struct. Chem. 2003, 14, 279–288. [Google Scholar] [CrossRef]
- Govender, T.; Kruger, H.G.; Makatini, M.; Onajole, O.K. Synthesis and NMR elucidation of pentacyclo-undecane diamine derivatives as potential anti-tuberculosis drugs. Struct. Chem. 2008, 19, 719–726. [Google Scholar] [CrossRef]
- Banister, S.D.; Moussa, I.A.; Jordan, M.J.T.; Coster, M.J.; Kassiou, M. Oxo-bridged isomers of aza-trishomocubane sigma (σ) receptor ligands: Synthesis, in vitro binding, and molecular modeling. Bioorg. Med. Chem. Lett. 2010, 20, 145–148. [Google Scholar] [CrossRef]
- Joubert, J.; Geldenhuys, W.J.; Van der Schyf, C.J.; Oliver, D.W.; Kruger, H.G.; Govender, T.; Malan, S.F. Polycyclic Cage Structures as Lipophilic Scaffolds for Neuroactive Drugs. Chem. Med. Chem. 2012, 7, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Cheekatla, S.R.; Meshram, M. Design and Synthesis of Cage Molecules as High Energy Density Materials for Aerospace Applications. ChemCatChem. 2020, 12, 6131–6172. [Google Scholar] [CrossRef]
- Gharpure, S.J.; Porwal, S.K. Synthesis of Oxa-, Aza- and Thia-Bowls and Cages. Org. Prep. Proc. Int. 2013, 45, 81–153. [Google Scholar] [CrossRef]
- Kotha, S.; Cheekatla, S.R.; Chinnam, A.K.; Jain, T. Design and synthesis of polycyclic bisindoles via Fischer indolization and ring-closing metathesis as key steps. Tetrahedron Lett. 2016, 57, 5605–5607. [Google Scholar] [CrossRef]
- Kotha, S.; Saifuddin, M.; Ali, R.; Sreevani, G. Spiro annulation of cage polycycles via Grignard reaction and ring-closing metathesis as key steps. Beilstein J. Org. Chem. 2015, 11, 1367–1372. [Google Scholar] [CrossRef]
- Kotha, S.; Cheekatla, S.R.; Mhatre, D.S. Ring-Closing Metathesis Approach to Cage Propellanes Containing Oxepane and Tetrahydrofuran Hybrid System. Synthesis 2017, 49, 5339–5350. [Google Scholar] [CrossRef]
- Kotha, S.; Manivannan, E. Synthesis of functionalized cis-syn-cis triquinanes. Indian J. Chem. Sect. B 2002, 41, 808–811. [Google Scholar]
- Kotha, S.; Cheekatla, S.R. Synthesis of cage [4.4.2]propellanes and D3-trishomocubanes bearing spiro linkage. J. Chem. Sci. 2018, 130, 171. [Google Scholar] [CrossRef]
- Mehta, G.; Rao, K.S. Reductive carbon-carbon cleavage in caged systems. A new general synthesis of linearly fused cis-syn-cis-triquinanes. J. Org. Chem. 1985, 50, 5537–5543. [Google Scholar] [CrossRef]
- Kotha, S.; Manivannan, E.; Sreenivasachary, N.; Ganesh, T.; Deb, A.C. Spiro-Annulation via Ring Closing Metathesis Reaction. Synlett. 1999, 1999, 1618–1620. [Google Scholar] [CrossRef]
- Kotha, S.; Krishna, N.G.; Halder, S.; Misra, S. A synergistic approach to polycyclics via a strategic utilization of Claisen rearrangement and olefin metathesis. Org. Biomol. Chem. 2011, 9, 5597–5624. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Halder, S. Ethyl Isocyanoacetate as a Useful Glycine Equivalent. Synlett 2010, 2010, 337–354. [Google Scholar] [CrossRef]
- Lannoye, G.; Kotha, S.; Wehrli, S.; Cook, J.M.; Weiss, U. General approach to the synthesis of polyquinenes via the Weiss reaction. 6. Progress toward the synthesis of dicyclopentapentalenes. J. Org. Chem. 1988, 53, 2327–2340. [Google Scholar] [CrossRef]
- Kotha, S.; Meshram, M.; Khedkar, P.; Banerjee, S.; Deodhar, D. Recent applications of ring-rearrangement metathesis in organic synthesis. Beilstein J. Org. Chem. 2015, 11, 1833–1864. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Shah, V.R.; Mandal, K. Formation of Arenes via Diallylarenes: Strategic Utilization of Suzuki–Miyaura Cross-Coupling, Claisen Rearrangement and Ring-Closing Metathesis. Adv. Synth. Catal. 2007, 349, 1159–1172. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotha, S.; Salman, M.; Cheekatla, S.R. Synthesis of Pentacycloundecane (PCUD) Based Spiro-Pyrano-Cage Framework via Ring-Closing Metathesis. Molbank 2023, 2023, M1567. https://doi.org/10.3390/M1567
Kotha S, Salman M, Cheekatla SR. Synthesis of Pentacycloundecane (PCUD) Based Spiro-Pyrano-Cage Framework via Ring-Closing Metathesis. Molbank. 2023; 2023(1):M1567. https://doi.org/10.3390/M1567
Chicago/Turabian StyleKotha, Sambasivarao, Mohammad Salman, and Subba Rao Cheekatla. 2023. "Synthesis of Pentacycloundecane (PCUD) Based Spiro-Pyrano-Cage Framework via Ring-Closing Metathesis" Molbank 2023, no. 1: M1567. https://doi.org/10.3390/M1567
APA StyleKotha, S., Salman, M., & Cheekatla, S. R. (2023). Synthesis of Pentacycloundecane (PCUD) Based Spiro-Pyrano-Cage Framework via Ring-Closing Metathesis. Molbank, 2023(1), M1567. https://doi.org/10.3390/M1567






