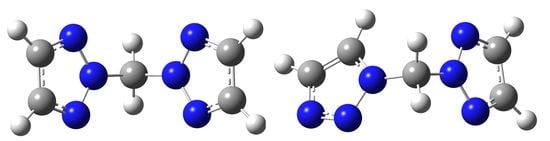
Synthesis of Bis(1,2,3-triazolyl)alkanes in Superbasic and Solvent-Free Conditions
Abstract
1. Introduction
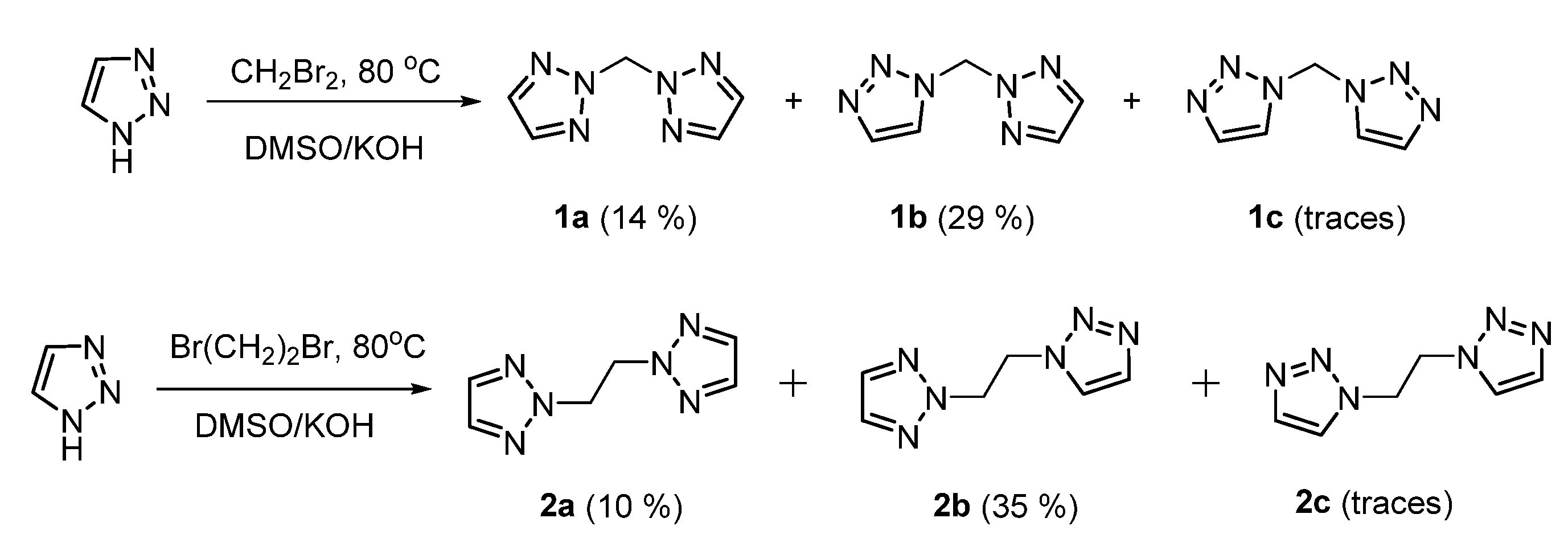
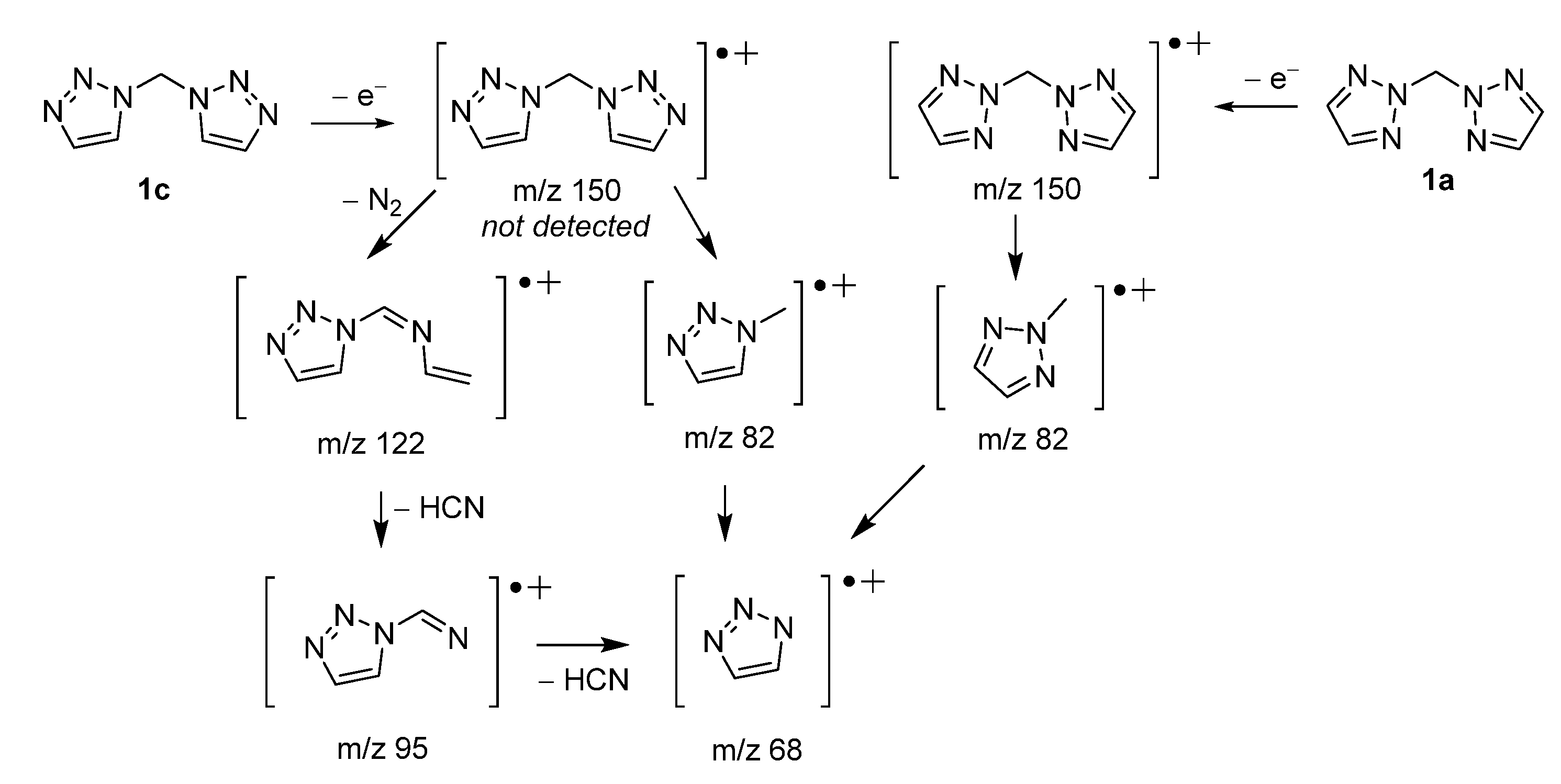
2. Results and Discussion
3. Materials and Methods
3.1. Instrumental Methods
3.2. Synthetic Procedures
3.2.1. Preparation of Bis(1,2,3-triazolyl)methane Isomers
3.2.2. Preparation of 1,2-Bis(1,2,3-triazolyl)ethane Isomers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-Triazoles as Pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef] [PubMed]
- Aromí, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Brunel, D.; Dumur, F. Recent advances in organic dyes and fluorophores comprising a 1,2,3-triazole moiety. New J. Chem. 2020, 44, 3546–3561. [Google Scholar] [CrossRef]
- Ahmed, F.; Xiong, H. Recent developments in 1,2,3-triazole-based chemosensors. Dye. Pigment. 2021, 185, 108905. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Zhao, Y. Click chemistry as a versatile reaction for construction and modification of metal-organic frameworks. Coord. Chem. Rev. 2019, 380, 484–518. [Google Scholar] [CrossRef]
- Agata, B.; Robert, B.; Joachim, K.; Marek, W.; Maciej, Z. How Nonequivalency of the Flexibility of the Ligand Bridges Leads to Anisotropy of Perturbation Transmission in a 3D Spin-Crossover Coordination Network. Eur. J. Inorg. Chem. 2012, 2013, 875–883. [Google Scholar]
- Bronisz, R. 1,4-Di(1,2,3-triazol-1-yl)butane as Building Block for the Preparation of the Iron(II) Spin-Crossover 2D Coordination Polymer. Inorg. Chem. 2005, 44, 4463–4465. [Google Scholar] [CrossRef]
- Białońska, A.; Bronisz, R.; Baranowski, Ł. 1D Spin-Crossover Networks Containing a Fe II (1,2,3-triazol-1-yl) 4 (CH 3 CN) 2 -Type Core. Eur. J. Inorg. Chem. 2013, 2013, 720–724. [Google Scholar] [CrossRef]
- Vereshchagin, L.I.; Tikhonova, L.G.; Maksikova, A.V.; Serebryakova, E.S.; Proidakov, A.G.; Filippova, T.M. Unsaturated carbonyl-containing compounds. XXVIII. Cycloaddition of organic azides to α-acetylene ketones and acids. Zhurnal Org. Khimii 1980, 16, 730–738. [Google Scholar] [CrossRef]
- Huo, J.Z.; Su, X.M.; Wu, X.X.; Liu, Y.Y.; Ding, B. Hydrothermal synthesis and characterization of a series of luminescent Ag(i) coordination polymers with two new multidentate bis-(1,2,3-triazole) ligands: Structural diversity, polymorphism and photoluminescent sensing. CrystEngComm 2016, 18, 6640–6652. [Google Scholar] [CrossRef]
- Kusz, J.; Bronisz, R.; Zubko, M.; Bednarek, G. On the Role of Intermolecular Interactions on Structural and Spin-Crossover Properties of 2D Coordination Networks [Fe(bbtr)3]A2 (bbtr=1,4-bis(1,2,3-triazol-1-yl)butane; A=ClO4−, BF4−). Chem. Eur. J. 2011, 17, 6807–6820. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Enachescu, C.; Humair, A.; Egger, L.; Delgado, T.; Tissot, A.; Guénée, L.; Besnard, C.; Bronisz, R.; Hauser, A. Light-induced spin-state switching in the mixed crystal series of the 2D coordination network {[Zn1−xFex(bbtr)3](BF4)2}∞: Optical spectroscopy and cooperative effects. Dalt. Trans. 2014, 43, 17786–17796. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Pillet, S.; Bendeif, E.-E.; Enachescu, C.; Bronisz, R.; Hauser, A. Light-Induced Bistability in the 2 D Coordination Network {[Fe(bbtr)3][BF4]2}∞: Wavelength-Selective Addressing of Molecular Spin States. Chem. Eur. J. 2013, 19, 11418–11428. [Google Scholar] [CrossRef]
- Bowden, K. Acidity Functions for Strongly Basic Solutions. Chem. Rev. 1966, 66, 119–131. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Vasil’tsov, A.M.; Amosova, S.V. Basicity of saturated solutions of alkali metal hydroxides in dimethylsulfoxide. Bull. Acad. Sci. USSR Div. Chem. Sci. 1986, 35, 682–686. [Google Scholar] [CrossRef]
- Gilchrist, T.L.; Gymer, G.E. 1,2,3-Triazoles. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Cambridge, MA, USA, 1974; Volume 16, pp. 33–85. [Google Scholar]
- Zdanovskaia, M.A.; Esselman, B.J.; Kougias, S.M.; Amberger, B.K.; Stanton, J.F.; Woods, R.C.; McMahon, R.J. Precise equilibrium structures of 1H- and 2H-1,2,3-triazoles (C2H3N3) by millimeter-wave spectroscopy. J. Chem. Phys. 2022, 157, 84305. [Google Scholar] [CrossRef]
- Semitut, E.Y.; Sukhikh, T.S.; Filatov, E.Y.; Anosova, G.A.; Ryadun, A.A.; Kovalenko, K.A.; Potapov, A.S. Synthesis, Crystal Structure, and Luminescent Properties of Novel Zinc Metal–Organic Frameworks Based on 1,3-Bis(1,2,4-triazol-1-yl)propane. Cryst. Growth Des. 2017, 17, 5559–5567. [Google Scholar] [CrossRef]
- Peresypkina, E.V.; Lider, E.V.; Smolentsev, A.I.; Sanchiz, J.; Gil-Hernández, B.; Potapov, A.S.; Khlebnikov, A.I.; Kryuchkova, N.A.; Lavrenova, L.G. Bis(benzotriazol-1-yl)methane as a linker in the assembly of new copper(II) coordination polymers: Synthesis, structure and investigations. Polyhedron 2012, 48, 253–263. [Google Scholar] [CrossRef]
- Marchenko, R.D.; Lysova, A.A.; Samsonenko, D.G.; Dybtsev, D.N.; Potapov, A.S. Synthesis, structural diversity, luminescent properties and antibacterial effects of cadmium(II) and silver(I) coordination compounds with bis(1,2,3-benzotriazol-1-yl)alkanes. Polyhedron 2020, 177, 114330. [Google Scholar] [CrossRef]
- Marchenko, R.; Potapov, A. 1,3-Bis(1,2,4-triazol-1-yl)adamantane. Molbank 2017, 2017, M968. [Google Scholar] [CrossRef]
- Cox, J.R.; Woodcock, S.; Hillier, I.H.; Vincent, M.A. Tautomerism of 1,2,3- and 1,2,4-triazole in the gas phase and in aqueous solution: A combined ab initio quantum mechanics and free energy perturbation study. J. Phys. Chem. 1990, 94, 5499–5501. [Google Scholar] [CrossRef]
- Albert, A.; Taylor, P.J. The tautomerism of 1,2,3-triazole in aqueous solution. J. Chem. Soc. Perkin Trans. 1989, 2, 1903–1905. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Venkataraghavan, R. Contribution to the infrared spectra of five-membered N- and N,S-heterocyclic compounds. Can. J. Chem. 1964, 42, 43–49. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchenko, R.D.; Potapov, A.S. Synthesis of Bis(1,2,3-triazolyl)alkanes in Superbasic and Solvent-Free Conditions. Molbank 2023, 2023, M1551. https://doi.org/10.3390/M1551
Marchenko RD, Potapov AS. Synthesis of Bis(1,2,3-triazolyl)alkanes in Superbasic and Solvent-Free Conditions. Molbank. 2023; 2023(1):M1551. https://doi.org/10.3390/M1551
Chicago/Turabian StyleMarchenko, Roman D., and Andrei S. Potapov. 2023. "Synthesis of Bis(1,2,3-triazolyl)alkanes in Superbasic and Solvent-Free Conditions" Molbank 2023, no. 1: M1551. https://doi.org/10.3390/M1551
APA StyleMarchenko, R. D., & Potapov, A. S. (2023). Synthesis of Bis(1,2,3-triazolyl)alkanes in Superbasic and Solvent-Free Conditions. Molbank, 2023(1), M1551. https://doi.org/10.3390/M1551