2,3-Bis((E)-4-hydroxybenzylidene)-N1,N4-bis(4-methylbenzyl)succinamide
Abstract
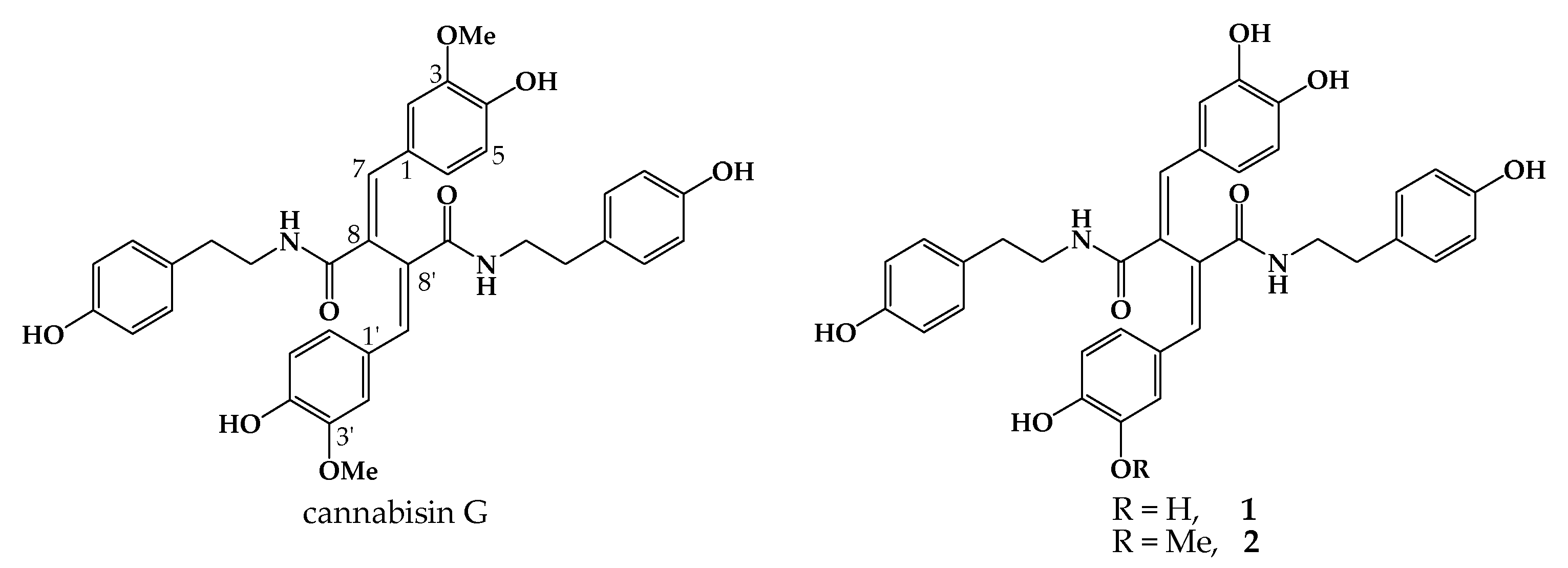
1. Introduction
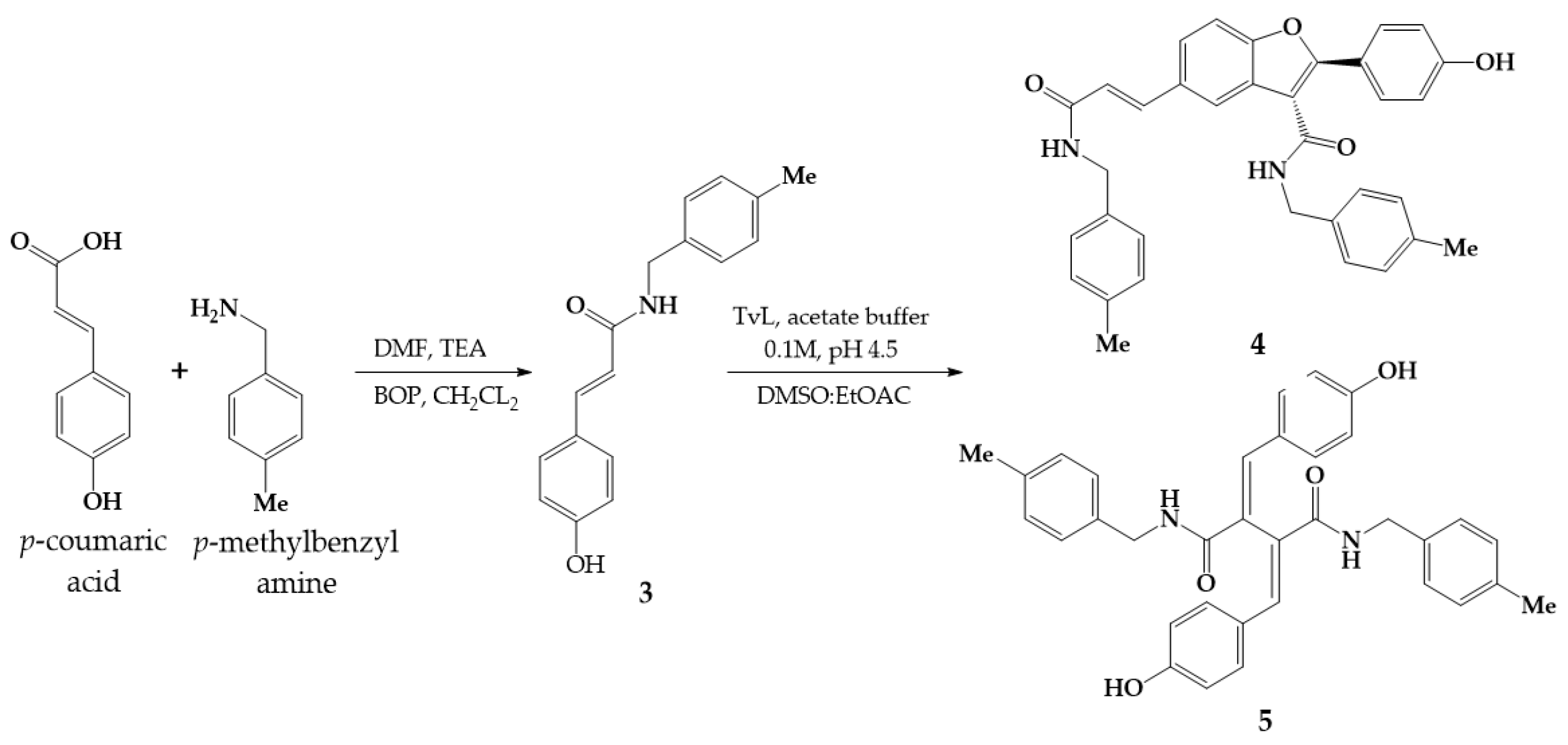
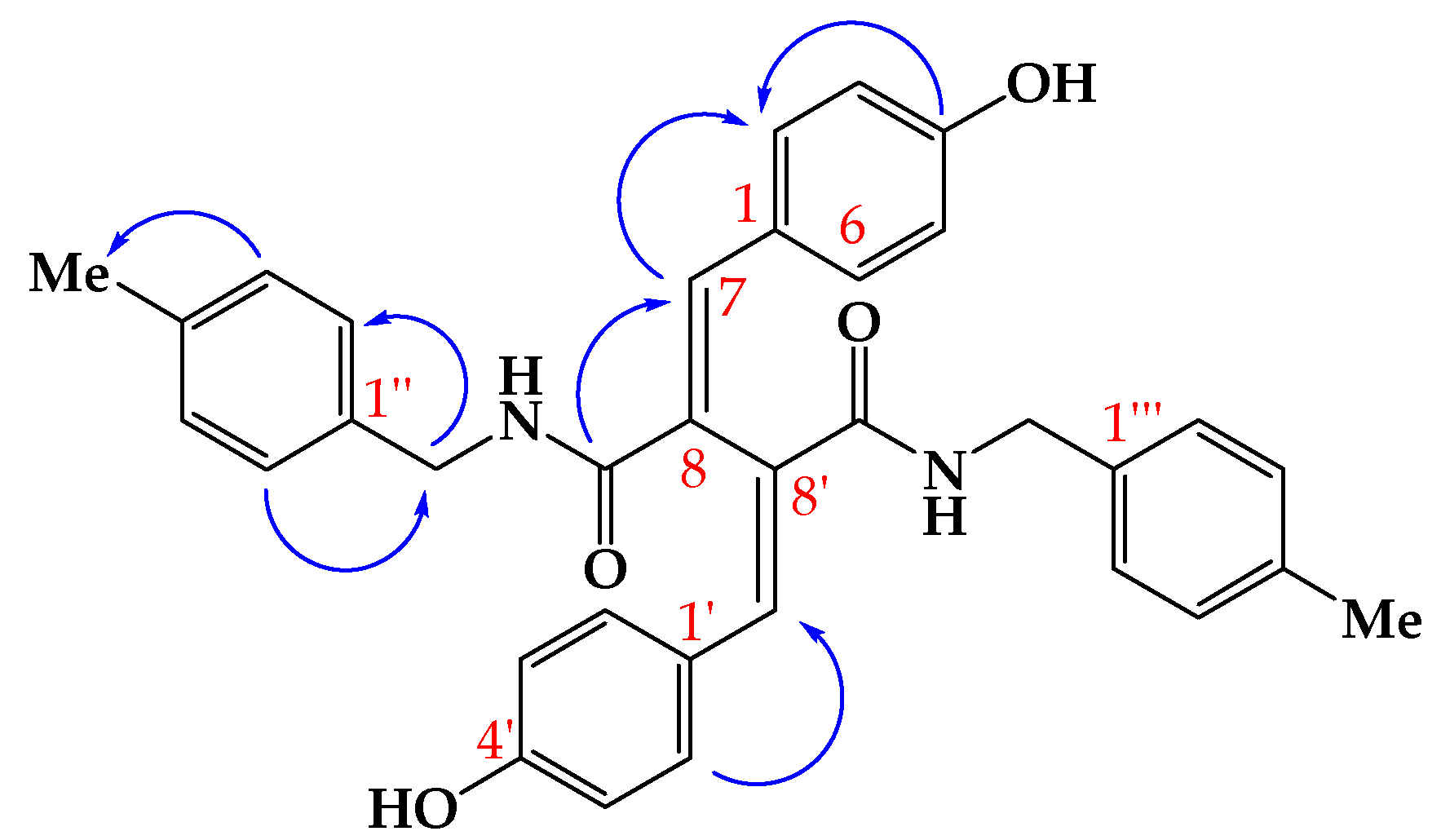
2. Results and Discussion
3. Materials and Methods
Synthesis of Lignanamide 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zalesak, F.; Bon, D.; Pospisil, J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef] [PubMed]
- Cardullo, N.; Muccilli, V.; Tringali, C. Laccase-mediated synthesis of bioactive natural products and their analogues. RSC Chem. Biol. 2022, 3, 614–647. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, I.; Ikeya, Y.; Hayashi, K.; Okada, M.; Maruno, M. Three acyclic bis-phenylpropane lignanamides from fruits of Cannabis Sativa. Phytochemistry 1995, 38, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Li, W.L.; Wang, Q.A.; Yang, Z.Q.; Hou, Z.J. Concise synthesis of Cannabisin G. Bioorg. Med. Chem. Lett. 2010, 20, 5095–5098. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gu, Y.-F.; Su, X.-Q.; Li, M.-M.; Huo, H.-X.; Zhang, J.; Zeng, K.-W.; Zhang, Q.; Zhao, Y.-F.; Li, J.; et al. Anti-inflammatory lignanamides from the roots of Solanum melongena L. Fitoterapia 2014, 98, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Tomosaka, H.; Chin, Y.W.; Salim, A.A.; Keller, W.J.; Chai, H.; Kinghorn, A.D. Antioxidant and cytoprotective compounds from Berberis vulgaris (barberry). Phytother. Res. 2008, 22, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Liu, W.K.; Che, C.T. Lignanamides and nonalkaloidal components of Hyoscyamus niger seeds. J. Nat. Prod. 2002, 65, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Lesma, G.; Consonni, R.; Gambaro, V.; Remuzzi, C.; Roda, G.; Silvani, A.; Vece, V.; Visconti, G.L. Cannabinoid-free Cannabis sativa L. grown in the Po valley: Evaluation of fatty acid profile, antioxidant capacity and metabolic content. Nat. Prod. Res. 2014, 28, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.F.; Zhao, Y.L.; Dai, Z.; Qin, X.J.; Yuan, H.L.; Jin, Q.; Wang, Y.F.; Liu, Y.P. Phenolic Amides with Immunomodulatory Activity from the Nonpolysaccharide Fraction of Lycium barbarum Fruits. J. Agric. Food Chem. 2020, 68, 4072. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.M.; Guo, Y.L.; Wen, Y.L. The total synthesis of cannabisin G. J. Serb. Chem. Soc. 2010, 75, 1617–1623. [Google Scholar] [CrossRef]
- Pulvirenti, L.; Muccilli, V.; Cardullo, N.; Spatafora, C.; Tringali, C. Chemoenzymatic Synthesis and alpha-Glucosidase Inhibitory Activity of Dimeric Neolignans Inspired by Magnolol. J. Nat. Prod. 2017, 80, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Cardullo, N.; Pulvirenti, L.; Spatafora, C.; Musso, N.; Barresi, V.; Condorelli, D.F.; Tringali, C. Dihydrobenzofuran Neolignanamides: Laccase-Mediated Biomimetic Synthesis and Antiproliferative Activity. J. Nat. Prod. 2016, 79, 2122–2134. [Google Scholar] [CrossRef] [PubMed]
- Mogharabi, M.; Faramarzi, M.A. Laccase and Laccase-Mediated Systems in the Synthesis of Organic Compounds. Adv. Synth. Catal. 2014, 356, 897–927. [Google Scholar] [CrossRef]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciacca, C.; Cardullo, N.; Muccilli, V. 2,3-Bis((E)-4-hydroxybenzylidene)-N1,N4-bis(4-methylbenzyl)succinamide. Molbank 2023, 2023, M1558. https://doi.org/10.3390/M1558
Sciacca C, Cardullo N, Muccilli V. 2,3-Bis((E)-4-hydroxybenzylidene)-N1,N4-bis(4-methylbenzyl)succinamide. Molbank. 2023; 2023(1):M1558. https://doi.org/10.3390/M1558
Chicago/Turabian StyleSciacca, Claudia, Nunzio Cardullo, and Vera Muccilli. 2023. "2,3-Bis((E)-4-hydroxybenzylidene)-N1,N4-bis(4-methylbenzyl)succinamide" Molbank 2023, no. 1: M1558. https://doi.org/10.3390/M1558
APA StyleSciacca, C., Cardullo, N., & Muccilli, V. (2023). 2,3-Bis((E)-4-hydroxybenzylidene)-N1,N4-bis(4-methylbenzyl)succinamide. Molbank, 2023(1), M1558. https://doi.org/10.3390/M1558





