Abstract
Reactions of 3-bromo-4-phenylisothiazole-5-carboxamide and 3-bromoisothiazole-5-carboxamide with NaNO2 (4 equiv.), in TFA, at ca. 0 °C gave the carboxylic acid products in 99% and 95% yields, respectively. The two compounds were fully characterized.
1. Introduction
Isothiazoles are useful compounds owing to their wide biological activity, industrial applications, and their use as synthetic intermediates [1,2]. An example of a biologically useful isothiazole is the antibacterial drug sulfasomizole, while other isothiazoles have been studied as anticancer agents [3], or showed acaricidal, insecticidal, and fungicidal activity [4,5]. Carboxylic acid substituted isothiazoles in particular have shown useful biological activity, such as the anti-HIV activity of 3-benzyloxyisothiazole-4/5-carboxylic acids 1 and 2 [6], hypolipidemic activity of phenoxy derivative 3 [7], and anti-inflammatory activity of carboxamide 4 [8] (Figure 1).
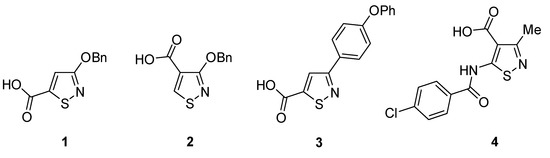
Figure 1.
Biologically active isothiazole carboxylates.
Our interest in isothiazoles focuses on their formation from 1,2,3-dithiazole precursors 5 by treatment with gaseous HCl or HBr [9] (Scheme 1). We later investigated the CH arylation of both the 5 [10] and the 4 [11] positions to obtain arylisothiazole products. Since both of these investigations involved mainly cyano-substituted isothiazoles, we looked at the possibility of functional group modifications by performing their hydration reaction in the presence of conc. H2SO4. Carboxamide products 9 and 10 were isolated in high yields [11] (Scheme 1).
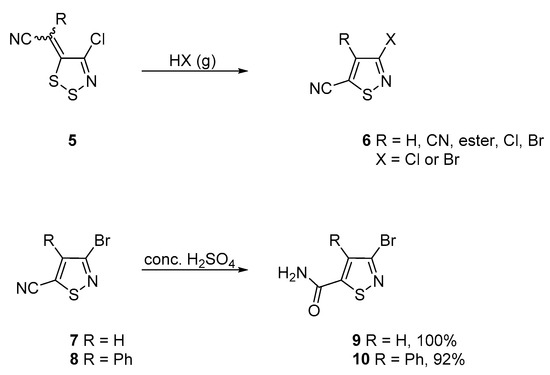
Scheme 1.
Route to isothiazole-5-carbonitriles 6 from dithiazoles 5 and synthesis of isothiazole-5-carboxamides 9 and 10.
As a continuation of the study on functional group modifications of these isothiazoles, we investigated the transformations of the carboxamide group to carboxylic acids. Similar transformations on isothiazole scaffolds were previously investigated by our team [12].
2. Results and Discussion
We started our investigation from 4-phenylisothiazole 10 by applying conditions used in the literature for a similar scaffold [12]. The reaction of 3-bromo-4-phenylisothiazole-5-carboxamide 10 with NaNO2 (10 equiv.), in conc. H2SO4, at ca. 100 °C led to a complete consumption of the starting isothiazole and isolation of the desired compound 11 in 39% yield (Scheme 2), while no other products were observed by TLC.
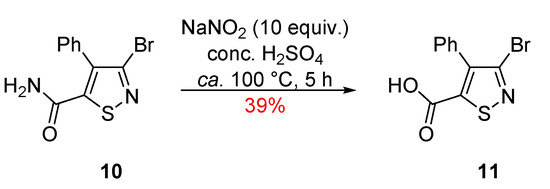
Scheme 2.
Synthesis of 3-bromo-4-phenylisothiazole-5-carboxylic acid (11).
Product 11 was isolated as colorless needles, mp 180–182 °C (from PhH). UV-vis spectroscopy in dichloromethane supported an intact isothiazole ring (λmax 298 nm, log ε 3.89). Mass spectrometry revealed a molecular ion (M+-H) peak of m/z 282 (87%) along with a M+-H++2 isotope peak at 284 (100%) that supported the presence of one bromine, while FTIR spectroscopy showed the presence of a broad carboxylic acid ν(O-H) stretch at 2926 cm−1 along with a strong ν(C=O) stretch at 1736 cm−1 compared to the 1672 cm−1 frequency of the amide carbonyl in the starting material 10 [11]. 13C NMR spectroscopy showed the presence of three aromatic CH resonances and five quaternary carbon resonances, with a downfield shift in the resonance of the carbonyl carbon from 160.7 ppm in the starting amide to 163.1 ppm for the carboxylic acid (see Supplementary materials for NMR spectra). Moreover, a correct elemental analysis (CHN) was obtained for the molecular formula C10H6BrNO2S.
In an attempt to improve the yield of carboxylic acid 11, we investigated a milder set of conditions that avoided the use of conc. H2SO4 as the solvent and required fewer equivalents of NaNO2 and a lower temperature [13]. The reaction of 4-phenylisothiazole 10 with NaNO2 (4 equiv.), in TFA, at ca. 0 °C led to a fast consumption of the starting isothiazole and isolation of the desired compound 11 in an excellent 99% yield (Scheme 3). The same reaction conditions were also applied to the reaction of 3-bromoisothiazole-5-carboxamide (9), which gave the desired carboxylic acid 12 in an excellent 95% yield (Scheme 3). Compound 12 has a PubChem number and is commercially available but no synthesis or data are reported on it.
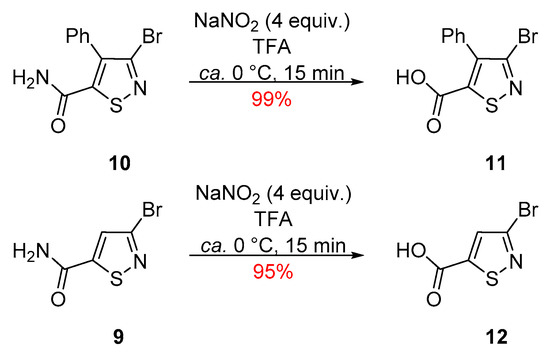
Scheme 3.
Synthesis of 3-bromo-4-phenylisothiazole-5-carboxylic acid (11) and 3-bromoisothiazole-5-carboxylic acid (12).
Product 12 was isolated as colorless plates, mp 139–141 °C (from c-hexane). UV-vis spectroscopy in dichloromethane supported an intact isothiazole ring (λmax 284 nm, log ε 3.37). Mass spectrometry revealed a molecular ion (MH+) peak of m/z 208 (97%) along with a MH++2 isotope peak at 210 (100%) that supported the presence of one bromine, while IR spectroscopy showed the presence of a broad carboxylic acid ν(O-H) stretch at 2874–2515 cm−1 along with strong ν(C=O) stretches at 1719 cm−1 compared to the 1674 cm−1 frequency of the amide carbonyl in the starting material 9 [11]. 13C NMR spectroscopy showed the presence of one CH resonance and three quaternary carbon resonances (see Supplementary materials for NMR spectra), while a correct elemental analysis (CHN) was obtained for the molecular formula C4H2BrNO2S.
Both isothiazole products 11 and 12 are multifunctional and potentially useful scaffolds for the synthesis of isothiazole derivatives.
3. Materials and Methods
The reaction mixture was monitored by TLC using commercial glass-backed thin layer chromatography (TLC) plates (Merck Kieselgel 60 F254). The plates were observed under a UV light at 254 and 365 nm. The melting point was determined using a PolyTherm-A, Wagner & Munz, Kofler—Hotstage Microscope apparatus (Wagner & Munz, Munich, Germany). The solvent used for recrystallization is indicated after the melting point. The UV-vis spectrum was obtained using a PerkinElmer Lambda-25 UV-vis spectrophotometer (PerkinElmer, Waltham, MA, USA) and inflections are identified by the abbreviation “inf”. The IR spectrum was recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer (Shimadzu, Kyoto, Japan) with a Pike Miracle Ge ATR accessory (Pike Miracle, Madison, WI, USA) and strong, medium and weak peaks are represented by s, m and w, respectively. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 machine [at 500 and 125 MHz, respectively, (Bruker, Billerica, MA, USA)]. Deuterated solvents were used for homonuclear lock and the signals are referenced to the deuterated solvent peaks. Attached proton test (APT) NMR studies were used for the assignment of the 13C peaks as CH3, CH2, CH and Cq (quaternary). MALDI-TOF mass spectra were recorded on a Bruker Autoflex III Smartbeam instrument. 3-Bromoisothiazole-5-carboxamide (9) and 3-bromo-4-phenylisothiazole-5-carboxamide (10) were prepared according to the literature procedure [11].
3.1. 3-Bromo-4-phenylisothiazole-5-carboxylic Acid (11)
To a stirred suspension of 3-bromo-4-phenylisothiazole-5-carboxamide (10) (56.6 mg, 0.20 mmol) in TFA (0.5 mL) cooled to ca. 0 °C was added NaNO2 (55.2 mg, 0.80 mmol) and the reaction mixture was stirred at this temperature until the consumption of the starting material (TLC, 15 min). The mixture was then poured onto water (5 mL) and extracted with t-BuOMe (3 × 10 mL), dried over Na2SO4 and evaporated to give the title compound 11 (56.2 mg, 99%) as colorless needles, mp 180–182 °C (from PhH); Rf 0.24 (t-BuOMe); (found: C, 42.21; H, 2.08; N, 4.93. C10H6BrNO2S requires C, 42.27; H, 2.13; N, 4.93%); λmax(DCM)/nm 298 (log ε 3.89); vmax/cm−1 2926 br (CO2H), 1736 s (C=O), 1530 w, 1485 w, 1443 w, 1393 m, 1348 w, 1329 w, 1213 s, 1179 m, 1153 m, 1088 w, 1074 w, 1034 w, 920 m, 887 w, 853 m, 799 m, 750 m; δH (500 MHz; CDCl3) 8.81 (1H, br s, CO2H), 7.49–7.43 (3H, m, Ar CH), 7.37–7.32 (2H, m, Ar CH); δC(125 MHz; CDCl3) 163.1 (Cq), 151.2 (Cq), 143.3 (Cq), 141.4 (Cq), 131.0 (Cq), 129.8 (CH), 129.2 (CH), 128.2 (CH); m/z (MALDI-TOF) 284 (81Br-M+–H, 100%), 282 (79Br-M+–H, 87), 271 (89), 264 (10).
3.2. 3-Bromoisothiazole-5-carboxylic Acid (12)
To a stirred suspension of 3-bromoisothiazole-5-carboxamide (9) (41.4 mg, 0.20 mmol), in TFA, (0.5 mL) cooled to ca. 0 °C was added NaNO2 (55.2 mg, 0.80 mmol) and the reaction mixture was stirred at this temperature until the consumption of the starting material (TLC, 15 min). The mixture was then poured onto water (5 mL) and extracted with t-BuOMe (3 × 10 mL), dried over Na2SO4 and evaporated to give the title compound 12 (39.6 mg, 95%) as colorless plates, mp 139–141 °C (from c-hexane); Rf 0.20 (t-BuOMe); (found: C, 22.75; H, 0.96; N, 7.08. C4H2BrNO2S requires C, 23.09; H, 0.97; N, 6.73%); λmax(DCM)/nm 284 (log ε 3.37); vmax/cm−1 3094 w (aryl C-H), 2874 w, 2754 w, 2706 w, 2567 w and 2515 w (CO2H), 1719s (C=O), 1653 w, 1558 w, 1512 m, 1420 w, 1364 m, 1315 s, 1260 m, 1240 m, 1215 s, 1140 m, 1067 w, 893 s, 878 m, 820 m, 752 s; δH (500 MHz; CDCl3+DMSO-d6) 7.52 (1H, s, Ar CH), 6.65 (1H, br. s, CO2H); δC (125 MHz; CDCl3+DMSO-d6) 160.3 (Cq), 160.1 (Cq), 137.0 (Cq), 129.5 (CH); m/z (MALDI-TOF) 210 (81Br-M++H, 100%), 208 (79Br-M++H, 97).
Supplementary Materials
The following supporting information can be downloaded at: mol file, 1H and 13C NMR and IR spectra.
Author Contributions
A.S.K. and P.A.K. conceived the experiments; A.S.K. designed the experiments; A.S.K. wrote the paper; A.S.K. and P.A.K. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Cyprus Research Promotion Foundation, grant numbers ΣΤΡAΤHΙΙ/0308/06, NEKYP/0308/02 ΥΓΕΙA/0506/19 and ΕΝΙΣΧ/0308/83.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the following organizations and companies in Cyprus for generous donations of chemicals and glassware: The State General Laboratory, the Agricultural Research Institute, the Ministry of Agriculture, MedoChemie Ltd. (Limassol, Cyprus), Medisell Ltd. (Nicosia, Cyprus) and Biotronics Ltd (Nicosia, Cyprus). Furthermore, we thank the A. G. Leventis Foundation for helping to establish the NMR facility at the University of Cyprus.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Clerici, F.; Gelmi, M.L.; Pellegrino, S.; Joule, J. Comprehensive Heterocyclic Chemistry III; Joule, J., Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Chapter 4.05; Volume 4, pp. 545–633. [Google Scholar]
- Potkin, V.I.; Kletskov, A.V.; Zubkov, F.I. Comprehensive Heterocyclic Chemistry IV; Aitken, R.A., Black, D.S., Cossy, J., Stevens, C.V., Eds.; Elsevier: Oxford, UK, 2022; Chapter 4.05; Volume 4, pp. 482–529. [Google Scholar]
- Larson, E.R.; Noe, M.C.; Gant, T.G. Isothiazole Derivatives Useful as Anticancer Agents. U.S. Patent 6,235,764, 22 May 2001. [Google Scholar]
- Hitoshi, S.; Yanase, Y.; Sekino, T.; Ishikawa, K.; Kuwatsuka, T.; Tanikawa, H.; Kawashima, H.; Tomura, N.; Kanemoto, Y.U.S. Isothiazolecarboxylic Acid Derivatives, Rice Blast Control Agents Containing the Same as Active Ingredients, and Rice Blast Control Method Applying the Control. Agents. Patent 5,240,951, 31 July 1993. [Google Scholar]
- Plant, A.; Boehmer, J.E.; Black, J.; Sparks, T.D. Isoxazolines Derivatives and Its Weedicide Application. CN Patent 101,052,628, 10 October 2007. [Google Scholar]
- Zeng, L.-F.; Zhang, H.-S.; Wang, Y.-H.; Sanchez, T.; Zheng, Y.-T.; Neamati, N.; Long, Y.-Q. Efficient synthesis and utilization of phenyl-substituted heteroaromatic carboxylic acids as aryl diketo acid isosteres in the design of novel HIV-1 integrase inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 4521–4524. [Google Scholar] [CrossRef] [PubMed]
- Tomisawa, K.; Kameo, K.; Matsunaga, T.; Saito, S.; Hosoda, K.; Asami, Y.; Sota, K. Studies on Hypolipidemic Agents. III: ω-(4-Phenoxybenzyl)-alkanoic Acid Derivatives. Chem. Pharm. Bull. 1986, 34, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Regiec, A.; Machon, Z.; Miedzybrodzki, R.; Szymaniec, S. New Isothiazole Derivatives: Synthesis, Reactivity, Physicochemical Properties and Pharmacological Activity. Arch. Pharm. 2006, 339, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Christoforou, I.C.; Ioannidou, H.A.; Manos, M.; Koutentis, P.A. Ring transformation of (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles to 3-haloisothiazole-5-carbonitriles. RSC Adv. 2013, 4, 7735–7748. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Koutentis, P.A. Silver-mediated palladium-catalyzed direct C-H arylation of 3-bromoisothiazole-4-carbonitrile. Org. Lett. 2011, 13, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. Silver mediated direct CH arylation of 3-bromoisothiazole-5-carbonitrile. Tetrahedron 2014, 70, 6796–6802. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A. 3,4,5-Triarylisothiazoles via C–C coupling chemistry. Org. Biomol. Chem. 2007, 5, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Britcher, S.F.; Lumma, J.; William, C. Diamino Isothiazole-1-oxides and 1,1-dioxides as Gastric Secretion Inhibitors. U.S. Patent 5,171,860, 15 December 1992. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).