Abstract
5H-Chromeno[2,3-b]pyridines are important compounds with industrial, biological, and medicinal properties. In this short note, the multicomponent reaction of salicylaldehyde, malononitrile dimer, and 2-phenyl-5-(trifluoromethyl)-2,4-dihydro-3H-pyrazol-3-one in dimethyl sulfoxide at ambient temperature was investigated to give 2,4-diamino-5-(5-hydroxy-1-phenyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile with good yield. The structure of the previously unknown chromeno[2,3-b]pyridine derivative was confirmed by elemental analysis, mass, nuclear magnetic resonance, and infrared spectroscopy data. The ADME (absorption, distribution, metabolism, and excretion) properties were also assessed.
1. Introduction
The assessment of absorption, distribution, metabolism, and excretion (ADME) properties is a necessary and responsible approach to drug discovery and design that aids in optimizing the safety of the hit compound [1]. Early estimation of ADME in the discovery phase drastically reduces the fraction of pharmacokinetics-related failures in clinical phases [2]. The high-throughput and low-cost nature of ADME prediction models allow for a more streamlined drug development process [3].
Chromeno[2,3-b]pyridine fragment is known as a privileged medicinal scaffold [4]. Its derivatives are important compounds with industrial, biological, and medicinal properties [5]. Chromeno[2,3-b]pyridines have a wide spectrum of pharmacological activity, and this moiety was found in two commercial anti-inflammatory drugs: Amlexanox [6] and Pranoprofen [7] (Figure 1).
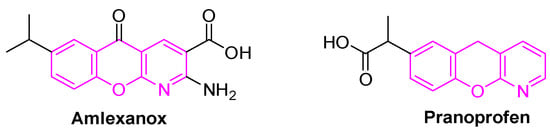
Figure 1.
Bioactive chromeno[2,3-b]pyridines.
The trifluoromethyl pyrazole fragment is a privileged medicinal scaffold as well. Many examples of bioactive fluorinated pyrazoles have emerged in recent years, including the non-steroidal anti-inflammatory drug celecoxib (Celebrex) [8] and the herbicide fluazolate [9] (Figure 2).
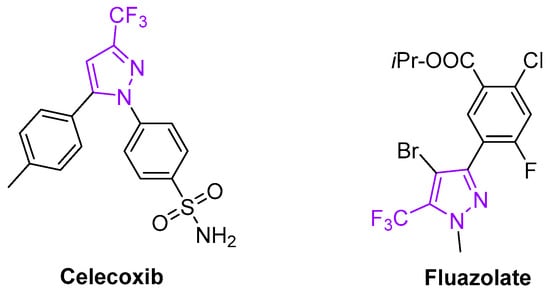
Figure 2.
Bioactive trifluoromethyl pyrazole derivatives.
The incorporation of both of these fragments may lead to new properties. Thus, the synthesis of a compound containing both of these fragments is of prominent interest.
2. Results and Discussion
2.1. Multicomponent Synthesis of 2,4-Diamino-5-(5-hydroxy-1-phenyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4
We previously carried out a multicomponent transformation of salicylaldehydes, 2-aminoprop-1-ene-1,1,3-tricarbonitrile (malononitrile dimer), and 5-(trifluoromethyl)-2,4-dihydro-3H-pyrazol-3-one into 2,4-diamino-5-(5-hydroxy-3-(trifluoromethyl)-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitriles by two different methods [10] (Scheme 1). However, the use of these reaction systems did not allow for the introduction of the N-phenyl-substituted C-H acid derivative into the reaction.
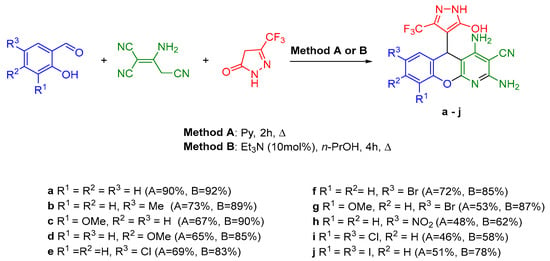
Scheme 1.
Multicomponent reaction of salicylaldehydes, malononitrile dimer, and 5-(trifluoromethyl)-2,4-dihydro-3H-pyrazol-3-one.
We developed the multicomponent synthesis of chromeno[2,3-b]pyridines in dimethyl sulfoxide (DMSO) [11,12,13]. This method produced chromeno[2,3-b]pyridines, which were previously unknown and unavailable via other methods (methods A and B [10]). Salicylaldehydes, malononitrile dimer, and malonic acid or dimethyl malonate were then transformed into 2-(2,4-diamino-3-cyano-5H-chromeno[2,3-b]-pyridin-5-yl)malonic acids or dimethyl 2-(2,4-diamino-3-cyano-5H-chromeno[2,3-b]-pyridin-5-yl)malonate [11,12]. Then, similarly, 2,4-Diamino-5-(nitromethyl)5H-chromeno[2,3-b]pyridine3-carbonitrile was also synthesized [13].
Now, we wish to report our results in the facile multicomponent transformation of salicylaldehyde (1), 2-aminoprop-1-ene-1,1,3-tricarbonitrile (2), and 2-phenyl-5-(trifluoromethyl)-2,4-dihydro-3H-pyrazol-3-one (3) into the previously unknown 2,4-diamino-5-(5-hydroxy-1-phenyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4) in DMSO at ambient temperature (23 °C, 24 h), as shown in Scheme 2.
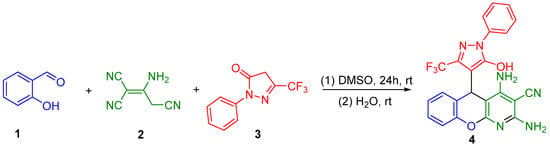
Scheme 2.
Reaction of salicylaldehyde (1), malononitrile dimer (2), and 2-phenyl-5-(trifluoromethyl)-2,4-dihydro-3H-pyrazol-3-one (3).
After the reaction was completed, water was poured into the DMSO solution, and the pure chromeno[2,3-b]pyridine (4) was precipitated. The yield of compound 4 was 84%.
The BFI (bond-forming index) [14] of the process is as high as four, as four new non-hydrogen bonds were formed in one synthetic transformation, namely two C-C bonds, one C-N bond, and one C-O bond.
The structure of the new chromeno[2,3-b]pyridine (4) was confirmed by spectral methods such as 1H and 13C NMR, IR spectroscopy and mass spectrometry data, and elemental analysis data (see Supplementary Materials). The NMR spectrum corresponds to similar known structures [10]. There is also a 2D NMR assignment for chromeno[2,3-b]pyridine aromatics [15].
Taking into consideration our previous results on the determination of intermediates [16] and 1H NMR monitoring data of similar multicomponent processes [11,17], the following mechanism for the transformation of salicylaldehyde (1), malononitrile dimer (2), and 2-phenyl-5-(trifluoromethyl)-2,4-dihydro-3H-pyrazol-3-one (3) was proposed, as shown in Scheme 3.
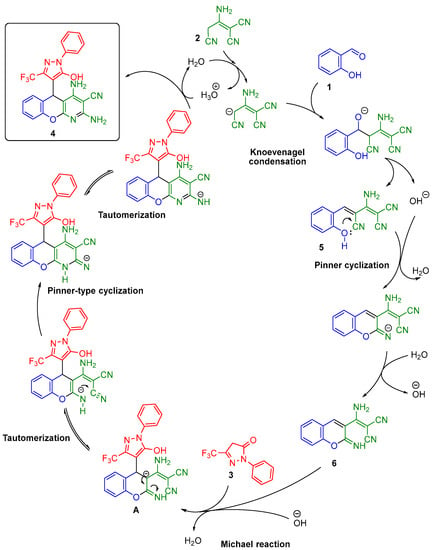
Scheme 3.
Simplified mechanism of salicylaldehyde (1), malononitrile dimer (2), and 2-phenyl-5-(trifluoromethyl)-2,4-dihydro-3H-pyrazol-3-one (3) transformation into chromeno[2,3-b]pyridine (4).
The first stage of the multicomponent transformation was a Knoevenagel condensation with the formation of unsaturated adduct 5 and the release of a hydroxide anion [18]. This hydroxide anion catalyzed a Pinner cyclization of adduct 5 into intermediate 6. The Michael addition of 2-phenyl-5-(trifluoromethyl)-2,4-dihydro-3H-pyrazol-3-one (3) then led to the formation of anion A. In the last stage, tautomerization, Pinner-type cyclization, and another tautomerization with protonation led to the final 2,4-diamino-5-(5-hydroxy-1-phenyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4).
2.2. ADME Prediction
The ADME of the synthesized chromeno[2,3-b]pyridine (4) was predicted using an online resource [19,20].
The bioavailability radar of chromeno[2,3-b]pyridine (4) is shown in Figure 3. Bioavailability radar allows for rapid assessment of drug similarity parameters [21]. Six physicochemical properties are taken into account: lipophilicity, size, polarity, solubility, flexibility, and saturation [22]. The pink area is the optimal range for each property. The synthesized compound (4) has a low boundary value of polarity but within the normal range of flexibility, as well as low saturation (Figure 3). Thus, compound 4 corresponds to Lipinski’s rule (Table 1) but has limited oral bioavailability.
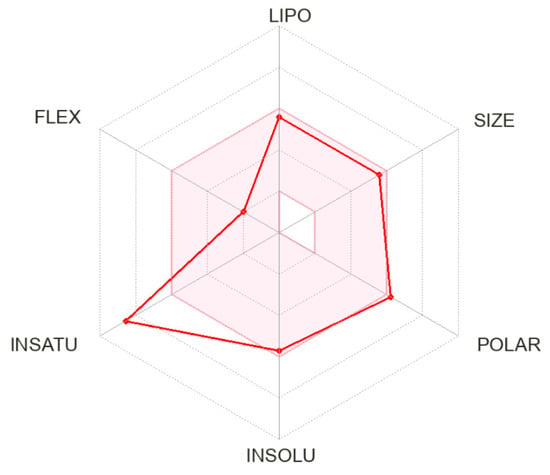
Figure 3.
The bioavailability radar of chromeno[2,3-b]pyridine (4).
The BOILED-Egg is a graphical display of two important parameters, i.e., passive gastrointestinal absorption (HIA) and brain access (BBB) [23]. The yolk is the physicochemical space for highly probable BBB permeation, and the white is the physicochemical space for highly probable HIA absorption. The two spaces are not mutually exclusive; the outside gray region stands for low absorption and limited brain penetration of the molecule. The point is supposed to be blue if the molecule is actively effluxed by P-glycoprotein (PGP+) and red (in this case) if it is a non-substrate of P-glycoprotein (PGP−) [24]. Figure 4 shows that the synthesized compound (4) is predicted not to be absorbed and not brain penetrant (outside the Egg), as well as not subject to active efflux (red dot).
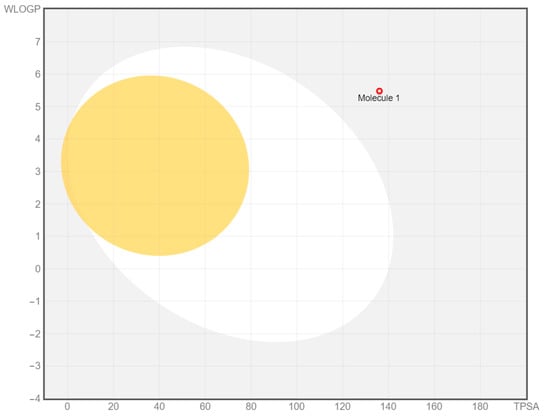
Figure 4.
The ‘BOILED-Egg’ diagram for chromeno[2,3-b]pyridine (4).
Some of the calculated ADME parameters are presented in Table 1. It also follows from the calculations that compound 4 complies with the Lipinski [25], Ghose [26], Veber [27], and Muegge [28] rules.

Table 1.
Calculated ADME parameters of synthesized chromeno[2,3-b]pyridine (4).
Table 1.
Calculated ADME parameters of synthesized chromeno[2,3-b]pyridine (4).
| Parameter | Value |
|---|---|
| Fraction Csp3 | 0.09 |
| Num. rotatable bonds | 3 |
| Topological polar surface area | 136.00 Å2 |
| Consensus Log Po/w (Lipophilicity) | 3.55 |
| Log S (ESOL) [29] | −5.72 |
| Water solubility | 8.88 × 10−4 mg/mL; 1.91 × 10−6 mol/L |
| Class | Moderately soluble |
| Gastrointestinal absorption | Low |
| BBB permeant | No |
| P-gp substrate | No |
| Log Kp (skin permeation) | −6.09 cm/s |
| Bioavailability score | 0.55 |
Based on the above data, it can be concluded that pyridine may be a potential drug.
3. Materials and Methods
3.1. General Methods
All solvents and reagents were used as received from commercial sources without further purification (except the reagents described below). 2-Aminoprop-1-ene-1,1,3-tricarbonitrile (2) (malononitrile dimer) was synthesized by dimerization of malononitrile in an alkaline medium [30]. 2-Phenyl-5-(trifluoromethyl)-2,4-dihydro-3H-pyrazol-3-one (3) was obtained from phenylhydrazine and ethyl 4,4,4-trifluoroacetoacetate according to the literature [31].
The melting point was measured with a Gallenkamp melting-point apparatus (Gallenkamp & Co., Ltd., London, UK). 1H and 13C NMR spectra were registered in DMSO-d6 with a Bruker AM300 spectrometer (Bruker Corporation, Billerica, MA, USA) at ambient temperature. The IR spectrum was recorded with a Bruker ALPHA-T FT-IR spectrometer (Bruker Corporation, Billerica, MA, USA) in KBr pellets. The MS spectrum (EI = 70 eV) was recorded with a Kratos MS-30 spectrometer (Kratos Analytical Ltd., Manchester, UK). For elemental analysis, a 2400 elemental analyzer (Perkin Elmer Inc., Waltham, MA, USA) was applied.
The ADME prediction of the synthesized molecule (4) was carried out using online resources [19,20].
3.2. Multicomponent Synthesis of 2,4-Diamino-5-(5-hydroxy-1-phenyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4
Salicylic aldehyde (1) (0.122 g, 1 mmol), 2-aminoprop-1-ene-1,1,3-tricarbonitrile (2) (0.132 g, 1 mmol), and 2-phenyl-5-(trifluoromethyl)-2,4-dihydro-3H-pyrazol-3-one (3) (0.228 g, 1 mmol) were stirred in 5 mL of DMSO for 24 h at ambient temperature (23 °C). After the process was finished, 10 mL of water was added to the reaction mixture. The precipitate was filtered, washed with well-chilled ethanol (3 mL × 2), and dried to isolate pure 2,4-diamino-5-(5-hydroxy-1-phenyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)-5H-chro-meno[2,3-b]pyridine-3-carbonitrile (4).
2,4-Diamino-5-(5-hydroxy-1-phenyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4). Yellowish powder; yield 84% (0.390 g); mp = 202–203 °C (decomp.) (from DMSO-H2O); FTIR (KBr) cm−1: 3557 (NH2), 3437 (NH2), 3342 (NH2), 3207 (NH2), 3069 (O-H), 2219 (C≡N), 1691 (C=N), 1647 (C=C Ar), 1600 (C-C Ar), 1536 (C=C Ar), 1494 (C=C Ar), 1473 (C=C Ar), 1454 (C=C Ar), 1280 (C-F), 1146 (C-F), 1120 (C-F). 1H NMR (300 MHz, DMSO-d6): δ 5.36 (s, 1H, CH), 6.02 (br s, 2H, NH2), 6.51 (br s, 2H, NH2), 7.07 (t, 3J = 8.1 Hz, 2H, 2 CH Ar s.ald.), 7.19-7.30 (m, 2H, 2 CH Ph), 7.43 (t, 3J = 7.2 Hz, 1H, CH Ph), 7.55 (t, 3J = 7.2 Hz, 2H, 2 CH Ph), 7.74 (d, 3J = 8.1 Hz, 2H, 2 CH Ar s.ald.), 11.52-12.78 (br s, 1H, OH) ppm; 13C NMR (75 MHz, DMSO-d6): δ 27.8 (C(5)H), 71.2 (C(3)-CN), 88.0 (C(4a)), 106.8 (C(4’)), 116.7 (C(9)H Ar), 116.8 (CN), 121.2 (q, 1J = 269.5 Hz, 1C, CF3), 122.9 (C(7)H Ar), 123.1 (2C, o-CH from Ph), 124.3 (p-CH from Ph), 128.0 (C(6)H Ar), 128.8 (C(8)H Ar), 129.7 (2C, m-CH from Ph), 137.2 (q, 2J = 37.1 Hz, 1C, C(3’)-CF3), 138.0 (2C, C(5a) and N(1’)-C), 149.8 (C(9a)), 150.9 (C(4)-NH2), 157.4 (C(2)-NH2), 158.9 (C(5’)-OH), 159.9 (C(1a)) ppm; MS (m/z, relative intensity %): 464 [M]+ (3), 444 [M − HF]+ (43), 277 [M − C8H6F3N2]+ (5), 237 [M − C10H6F3N2O]+ (100), 228 [C10H7F3N2O]+ (47), 77 [C6H5]+ (31); Anal. calcd. for C23H15F3N6O2: C, 59.48; H, 3.26; N, 18.10%; found: C, 59.40; H, 3.36; N, 18.05%.
1H and 13C NMR, IR and MS spectra for compound 4 are presented in the Supplementary Materials.
4. Conclusions
In conclusion, the title compound, 2,4-diamino-5-(5-hydroxy-1-phenyl-3-(trifluo-romethyl)-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile, was synthesized in good yield using a facile and efficient multicomponent reaction. During the study, we used simple equipment and available starting materials. The newly synthesized compound was characterized by spectroscopic techniques (NMR, IR, and MS-EI) and elemental analysis. According to ADME parameters, the resulting chromo[2,3-b]pyridine contains two pharmacologically promising fragments and has a chance of being useful as a drug.
Supplementary Materials
The following are available online: compound 4 spectra: 1H NMR (Figure S1), 13C NMR (Figure S2), IR (Figure S3), and MS (EI) (Figure S4).
Author Contributions
Conceptualization, F.V.R. and M.N.E.; methodology, F.V.R. and Y.E.R.; software, F.V.R.; validation, Y.E.R., F.V.R., and M.N.E.; investigation, Y.E.R., F.V.R., and O.I.M.; resources, M.N.E.; data curation, Y.E.R.; writing—original draft preparation, Y.E.R.; writing—review and editing, F.V.R. and M.N.E.; supervision, M.N.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for the compound presented in this study are available in the Supplementary Materials of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pantaleão, S.Q.; Fernandes, P.O.; Gonçalves, J.E.; Maltarollo, V.G.; Honorio, K.M. Recent Advances in the Prediction of Pharmacokinetics Properties in Drug Design Studies: A Review. ChemMedChem 2022, 17, e202100542. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, J.; Xu, Y.; Zhou, N.; Peng, J.; Xiong, Z.; Liu, X.; Luo, X.; Luo, C.; Chen, K.; et al. In silico ADME/T modelling for rational drug design. Q. Rev. Biophys. 2015, 48, 488–515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dietrich, J. Privileged scaffolds in lead generation. Expert Opin. Drug Discov. 2015, 10, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Elinson, M.N.; Ryzhkova, Y.E.; Ryzhkov, F.V. Multicomponent design of chromeno[2,3-b]pyridine systems. Russ. Chem. Rev. 2021, 90, 94–115. [Google Scholar] [CrossRef]
- Makino, H.; Saijo, T.; Ashida, Y.; Kuriki, H.; Maki, Y. Mechanism of Action of an Antiallergic Agent, Amlexanox (AA-673), in Inhibiting Histamine Release from Mast Cells. Int. Arch. Allergy Immunol. 1987, 82, 66–71. [Google Scholar] [CrossRef]
- Akyol-Salman, İ.; Leçe-Sertöz, D.; Baykal, O. Topical Pranoprofen 0.1% Is As Effective Anti-Inflammatory and Analgesic Agent as Diclofenac Sodium 0.1% After Strabismus Surgery. J. Ocul. Pharmacol. Ther. 2007, 23, 280–283. [Google Scholar] [CrossRef]
- Penning, T.D.; Talley, J.J.; Bertenshaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Malecha, J.W.; Miyashiro, J.M.; et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: Identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef]
- Maxwell, B.D. The radiolabeled syntheses of JV 485, a herbicide candidate for winter wheat. J. Label. Compd. Radiopharm. 2000, 43, 645–654. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. A facile and efficient multicomponent approach to 5-[5-hydroxy-3-(trifluoromethyl)-1H-pyrazol-4-yl]-5H-chromeno[2,3-b]pyridines. J. Fluor. Chem. 2018, 213, 31–36. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Elinson, M.N.; Maslov, O.I.; Fakhrutdinov, A.N. Multicomponent Synthesis of 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonic Acids in DMSO. Molecules 2021, 26, 6839. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkova, Y.E.; Maslov, O.I.; Elinson, M.N. Dimethyl 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonate. Molbank 2022, 2022, M1308. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Ryzhkov, F.V.; Maslov, O.I.; Elinson, M.N. 2,4-Diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile. Molbank 2022, 2022, M1365. [Google Scholar] [CrossRef]
- Domling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkova, Y.E.; Ryzhkov, F.V.; Elinson, M.N.; Maslov, O.I.; Fakhrutdinov, A.N. One-Pot Solvent-Involved Synthesis of 5-O-Substituted 5H-Chromeno[2,3-b]pyridines. Molecules 2023, 28, 64. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. Pot-, Atom- and Step-Economic (PASE) Multicomponent approach to the 5-(Dialkylphosphonate)-Substituted 2,4-Diamino-5H-chromeno-[2,3-b]pyridine scaffold. Eur. J. Org. Chem. 2019, 2019, 4171–4178. [Google Scholar] [CrossRef]
- Ryzhkov, F.V.; Ryzhkova, Y.E.; Elinson, M.N.; Vorobyev, S.V.; Fakhrutdinov, A.N.; Vereshchagin, A.N.; Egorov, M.P. Catalyst-Solvent System for PASE Approach to Hydroxyquinolinone-Substituted Chromeno[2,3-b]pyridines Its Quantum Chemical Study and Investigation of Reaction Mechanism. Molecules 2020, 25, 2573. [Google Scholar] [CrossRef]
- Patai, S.; Israeli, Y. 411. The kinetics and mechanisms of carbonyl–methylene condensations. Part VII. The reaction of malononitrile with aromatic aldehydes in ethanol. J. Chem. Soc. 1960, 2025–2030. [Google Scholar] [CrossRef]
- Prediction of ADME Parameters, Pharmacokinetic Properties, Druglike Nature and Medicinal Chemistry Friendliness of One or Multiple Small Molecules to Support Drug Discovery. Available online: http://www.swissadme.ch (accessed on 31 October 2022).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ritchie, T.J.; Ertl, P.; Lewis, R. The graphical representation of ADME-related molecule properties for medicinal chemists. Drug Discov. Today 2011, 16, 65–72. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.; Kimchi-Sarfaty, C.; Sauna, Z.; Gottesman, M.M. P-glycoprotein: From genomics to mechanism. Oncogene 2003, 22, 7468–7485. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 12, 1841–1846. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating aqueous solubility directly from molecular structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Mittelbach, M. An improved and facile synthesis of 2-amino-1,1,3-tricyanopropene. Monatsh. Chem. 1985, 116, 689–691. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Zhang, K.; Chen, J.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. An efficient and highly stereoselective synthesis of novel trifluoromethylated trans-dihydrofuro[2,3-c]pyrazoles using arsonium ylides. Tetrahedron 2012, 68, 2121–2127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).