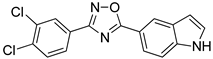
Abstract
3-(3,4-Dichlorophenyl)-5-(1H-indol-5-yl)-1,2,4-oxadiazole was synthesized via the condensation of 3,4-dichlorobenzamidoxime and methyl 1H-indole-5-carboxylate using a superbasic medium (NaOH/DMSO). The compound was tested as a potential inhibitor of human monoamine oxidase (MAO) A and B. It demonstrated a notable inhibition with an IC50 value of 0.036 μM for the MAO-B and isoform specificity. The product was characterized by 1H-NMR, 13C-NMR, and HRMS. In conclusion, the new active MAO-B inhibitor may serve as a candidate for the future discovery of therapeutic agents for neurodegenerative disorders such as Parkinson’s disease.
1. Introduction
Parkinson’s disease (PD) is an age-related neurodegenerative disorder of the central nervous system (CNS), currently affecting over 8% of individuals aged 65 years and older worldwide. PD results in the chronic, irreversible, and progressive neuronal degradation of specific areas in the human brain and is caused by complex pathophysiological processes, including oxidative stress, neuro-inflammation, excitotoxicity, mitochondrial dysfunction, and proteolytic stress [1,2,3].
Monoamine oxidases (MAOs) are mitochondrial flavoenzymes that play a key role in the metabolism of monoaminergic neurotransmitters. The selective inhibition of MAO-B is a well-established approach in the treatment of PD [4,5,6]. For example, the irreversible MAO-B inhibitor, selegiline, and the reversible inhibitor, safinamide, have been approved for the treatment of PD [4,5,6] (Figure 1a).
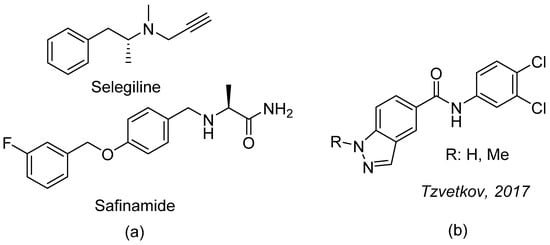
Figure 1.
(a) Structures of MAO-B-specific inhibitors; (b) the series of indole and indazole derived MAO inhibitors.
Despite the diversity of MAO-B inhibitors that has been described in the literature, the discovery of novel inhibitors with good potencies and isoform specificities is still of interest. Recently, indole and indazole derivatives have been reported to be highly potent and specific MAO-B inhibitors (Figure 1b) [7]. However, the amide bond in the central fragment of these compounds may be metabolically labile and could lead to the rapid inactivation of future drugs in this class.
Our research group has reported a variety of new lead compounds for the discovery of isoform-specific MAO-B inhibitors such as imidazolines, benzenesulfonamides, and pyrazolo[1,5-a]quinoxalin-4-ones [8,9,10]. Based on our interest in the discovery of novel MAO-B inhibitors, in this work, we used our previously developed synthetic approach to synthesize a new indole-derivative where the amide functionality has been replaced by a bioisostere.
According to the data of the Swiss Institute of Bioinformatics, the central amide group of the molecule can be replaced without a loss of biological activity by the 1,2,4-oxadiazole heterocyclic fragment. This substitution should have a favorable effect on the in vivo metabolic stability of drug leads due to the greater stability of the fragments of five-membered heterocycles compared to the amide bond.
In addition, indole- and oxadiazole-based building blocks and their composition and complexes are popular chemical tools for the creation of new biologically active compounds with anti-infective [11], anticancer [12], cytoprotective activity [13], and other biological properties [14].
Thus, we hypothesize that the novel 1,2,4-oxadiazole/indole hybrid framework described below may appeal to scientists as a starting point for the new drug candidate development.
2. Results
2.1. Chemistry
3-(3,4-Dichlorophenyl)-5-(1H-indol-5-yl)-1,2,4-oxadiazole (4) was synthesized by the condensation of amidoxime 2 with carboxylic acid ester 3 in the superbasic medium (NaOH/DMSO) (Scheme 1), as described in our previous work [15]. The amidoxime 2 was synthesized from the corresponding nitrile 1, as described in the literature [16].
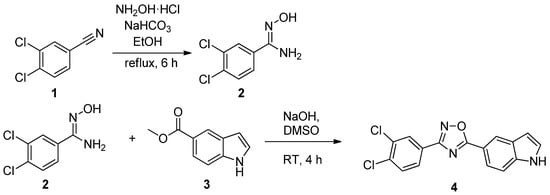
Scheme 1.
Synthesis of 3,4-dichlorobenzamidoxime 2 and 3-(3,4-dichlorophenyl)-5-(1H-indol-5-yl)-1,2,4-oxadiazole 4.
2.2. MAO Inhibition
The MAO inhibition potency of 3-(3,4-dichlorophenyl)-5-(1H-indol-5-yl)-1,2,4-oxadiazole was investigated using recombinant human MAO-A and MAO-B, following the same protocol as described for the previous investigation of 1,3,4-oxadiazol-2-ylbenzenesulfonamides [8]. The results of the MAO inhibition studies are presented in Table 1. Compound 4 inhibited MAO-B with an IC50 value of 0.036 μM, whereas a weak inhibition was recorded for MAO-A.

Table 1.
The inhibition of human MAO-A and MAO-B by 3-(3,4-dichlorophenyl)-5-(1H-indol-5-yl)-1,2,4-oxadiazole 4.
3. Discussion
This study reports the MAO inhibition potency of 3-(3,4-dichlorophenyl)-5-(1H-indol-5-yl)-1,2,4-oxadiazole (4). This compound inhibited MAO-B with an IC50 value of 0.036 μM, while the MAO-A isoform was inhibited with an IC50 value of 150 μM. The discovery of this active MAO-B inhibitor paves the way for the future discovery of potent MAO-B inhibitors among indole derivatives, which may find an application in the treatment of neurodegenerative disorders such as PD.
4. Materials and Methods
4.1. General
All the reagents and solvents were obtained from commercial sources and were used without purification. The reactions were monitored by analytical thin layer chromatography (TLC) using Silufol-254 plates. The visualization of the developed plates was performed by fluorescence quenching at 254 nm. 1H-NMR and 13C-NMR spectra were recorded on a Varian 400 Unity Plus instrument (400 MHz for 1H and 100 MHz for 13C, respectively). Chemical shifts (δ) are given in parts per million (ppm) and were referenced to the solvent signal for DMSO-d6 (2.50 for proton and 39.52 for carbon), while the coupling constants (J) are reported in hertz (Hz). Multiplicities are abbreviated as follows: s = singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quartet, and m = multiplet. The melting points were determined on an Electrothermal IA 9300 series digital melting point apparatus. The mass spectra were recorded on microTOF spectrometers (ESI ionization).
4.2. Synthesis and Characterization of 3-(3,4-Dichlorophenyl)-5-(1H-indol-5-yl)-1,2,4-oxadiazole 4
To a solution of 3,4-dichlorobenzamidoxime 2 (0.0015 mol, 1 equiv.) and methyl 1H-indole-5-carboxylate 3 (0.0015 mol, 1 equiv.) in DMSO (1 mL), powdered NaOH (0.002 mol, 1.3 equiv.) was rapidly added. The reaction mixture was stirred at room temperature for the required time (TLC). The reaction mixture was diluted with cold water (30–50 mL). The resulting precipitate was collected by filtration, washed with water (30 mL), and air-dried at 50 °C. The yield was 0.391g, 79%, beige solid, mp 193–195 °C; 1H-NMR (400 MHz, DMSO) δ 11.64 (s, 1H), 8.45 (s, 1H), 8.21 (d, J = 2.1 Hz, 1H), 8.03 (dd, J = 8.4, 2.0 Hz, 1H), 7.91–7.82 (m, 2H), 7.62 (d, J = 8.5 Hz, 1H), 7.54 (d, J = 3.2 Hz, 1H), 6.66 (d, J = 3.2 Hz, 1H); 13C-NMR (101 MHz, DMSO) δ 178.05, 167.05, 139.17, 134.86, 132.79, 132.32, 129.32, 128.49, 127.74, 122.09, 121.25, 114.51, 113.17, 103.41; MS (ESI+): m/z [M + H]+. Anal. Calcd for C16H10Cl2N3O: 330.0196. Found: 330.0182. 1H-NMR, 13C-NMR of compound 4 are presented in Supplementary Materials.
4.3. Synthesis and Characterization of 3,4-Dichlorobenzamidoxime 2
To a suspension of 3,4-dichlorobenzonitrile (0.012 mol, 1 equiv.) and NH2OH·HCl (0.018 mol, 1.5 equiv.) in ethanol (15 ml), NaHCO3 (0.018 mol, 1.5 equiv.) was added. The reaction mixture was heated under reflux for 2 h (TLC). The solvent was subsequently evaporated under a reduced pressure, and the reaction mixture was diluted with cold water. The resulting precipitate was collected by filtration, washed with water (30 mL), and air-dried at 50 °C. The yield was 86%, white solid, mp 143–145 °C; 1H-NMR (400 MHz, DMSO) δ 9.87 (s, 1H), 7.89 (d, J = 1.9 Hz, 1H), 7.71–7.60 (m, 2H), 5.95 (s, 2H). [18]
4.4. MAO Inhibition Studies
The measurement of the IC50 values for the inhibition of human MAO-A and MAO-B was carried out according to the previously reported protocols [8,19]. Recombinant human MAO-A and MAO-B were obtained from Sigma-Aldrich (St. Louis, MO, USA) and the fluorescence measurements were rescored with a SpectraMax iD3 Multi-Mode microplate reader (Molecular Devices). The measurement of the MAO activity was based on the fluorescence signal generated when the substrate, kynuramine, is oxidized by the MAOs to yield 4-hydroxyquinoline.
Supplementary Materials
The following supporting information can be downloaded online, Copies of 1H- and 13C-NMR spectra.
Author Contributions
The conceptualization of the study was done by A.A.S. and S.V.B.; the formal analysis was done by S.V.B.; formal analysis and investigation—J.A.E. and A.P.; writing—original draft preparation was done by J.A.E.; writing—review and editing was done by A.A.S. and J.P.P. All authors have read and agreed to the published version of the manuscript.
Funding
Chemical part of this work was supported by the Russian Science Foundation (project 22-13-20085). MAO inhibition studies were funded by the National Research Foundation of South Africa [grant specific unique reference numbers (UID) 137997 (JPP) and 132168 (AP)].
Data Availability Statement
Not applicable.
Acknowledgments
The grant holders acknowledge that the opinions, findings, and conclusions or recommendations expressed in any publication which is NRF-supported are that of the authors, and that the NRF accepts no liability whatsoever in this regard.
Conflicts of Interest
The authors declare no competing interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Santos, M.A.; Chand, K.; Chaves, S. Recent progress in multifunctional metal chelators as potential drugs for Alzheimer’s disease. Coord. Chem. Rev. 2016, 327, 287–303. [Google Scholar] [CrossRef]
- Meredith, G.E.; Totterdell, S.; Beales, M.; Meshul, C.K. Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson’s disease. Exp. Neurol. 2009, 219, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Belaidi, A.A.; Bush, A.I. Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: Targets for therapeutics. J. Neurochem. 2016, 139 (Suppl. 1), 179–197. [Google Scholar] [CrossRef]
- Fowler, J.S.; Logan, J.; Volkow, N.D.; Shumay, E.; McCall-Perez, F.; Jayne, M.; Wang, G.-J.; Alexoff, D.L.; Apelskog-Torres, K.; Hubbard, B.; et al. Evidence that formulations of the selective MAO-B inhibitor selegiline, which bypass first-pass metabolism, also inhibit MAO-A in the human brain. Neuropsychopharmacology 2015, 40, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Sheetal; Mantha, A.K.; Kumar, V. Recent developments on the structure-activity relationship studies of MAO inhibitors and their role in different neurological disorders. RSC Adv. 2016, 6, 42660–42683. [Google Scholar] [CrossRef]
- Fabbri, M.; Rosa, M.M.; Abreu, D.; Ferreira, J.J. Clinical pharmacology review of safinamide for the treatment of Parkinson’s disease. Neurodegener. Dis. Manag. 2015, 5, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkov, N.T.; Antonov, L. Subnanomolar indazole-5-carboxamide inhibitors of monoamine oxidase B (MAO-B) continued: Indications of iron binding, experimental evidence for optimised solubility and brain penetration. J. Enzyme Inhib. Med. Chem. 2017, 32, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Shetnev, A.; Shlenev, R.; Efimova, J.; Ivanovskii, S.; Tarasov, A.; Petzer, A.; Petzer, J.P. 1,3,4-Oxadiazol-2-ylbenzenesulfonamides as privileged structures for the inhibition of monoamine oxidase B. Bioorg. Med. Chem. Lett. 2019, 29, 126677. [Google Scholar] [CrossRef] [PubMed]
- Shetnev, A.; Osipyan, A.; Baykov, S.; Sapegin, A.; Chirkova, Z.; Korsakov, M.; Petzer, A.; Engelbrecht, I.; Petzer, J.P. Novel monoamine oxidase inhibitors based on the privileged 2-imidazoline molecular framework. Bioorg. Med. Chem. Lett. 2019, 29, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Panova, V.A.; Filimonov, S.I.; Chirkova, Z.V.; Kabanova, M.V.; Shetnev, A.A.; Korsakov, M.K.; Petzer, A.; Petzer, J.P.; Suponitsky, K.Y. Investigation of pyrazolo[1,5-a]quinoxalin-4-ones as novel monoamine oxidase inhibitors. Bioorg. Chem. 2021, 108, 104563. [Google Scholar] [CrossRef] [PubMed]
- Dhameliya, T.M.; Chudasma, S.J.; Patel, T.M.; Dave, B.P. A Review on Synthetic Account of 1,2,4-Oxadiazoles as Anti-Infective Agents. Mol. Divers. 2022, 26, 2967–2980. [Google Scholar] [CrossRef] [PubMed]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Xu, L.-L.; Zhu, J.-F.; Xu, X.-L.; Zhu, J.; Li, L.; Xi, M.-Y.; Jiang, Z.-Y.; Zhang, M.-Y.; Liu, F.; Lu, M.; et al. Discovery and Modification of in Vivo Active Nrf2 Activators with 1,2,4-Oxadiazole Core: Hits Identification and Structure–Activity Relationship Study. J. Med. Chem. 2015, 58, 5419–5436. [Google Scholar] [CrossRef] [PubMed]
- Street, L.J.; Baker, R.; Castro, J.L.; Chambers, M.S.; Guiblin, A.R.; Hobbs, S.C.; Matassa, V.G.; Reeve, A.J.; Beer, M.S.; Middlemiss, D.N. Synthesis and serotonergic activity of 5-(oxadiazolyl)tryptamines: Potent agonists for 5-HT1D receptors. J. Med. Chem. 1993, 36, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Baykov, S.; Sharonova, T.; Shetnev, A.; Rozhkov, S.; Kalinin, S.; Smirnov, A.V. The first one-pot ambient-temperature synthesis of 1,2,4-oxadiazoles from amidoximes and carboxylic acid esters. Tetrahedron 2017, 73, 945–951. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Pereira, M.C.; Faustino, W.W.M.; Coutinho, K.; dos Anjos, J.V.; de Melo, S.J. Synthesis, mechanism of for-mation, and molecular orbital calculations of arylamidoximes. Monatsh. Chem. 2009, 140, 1319–1324. [Google Scholar] [CrossRef]
- Petzer, A.; Pienaar, A.; Petzer, J.P. The inhibition of monoamine oxidase by esomeprazole. Drug Res. 2013, 63, 462–467. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Kasibhatla, S.; Kuemmerle, J.; Kemnitzer, W.; Ollis-Mason, K.; Qiu, L.; Grundy, C.C.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery and Structure−Activity Relationship of 3-Aryl-5-aryl-1,2,4-oxadiazoles as a New Series of Apoptosis Inducers and Potential Anticancer Agents. J. Med. Chem. 2005, 48, 5215–5223. [Google Scholar] [CrossRef] [PubMed]
- Mostert, S.; Petzer, A.; Petzer, J.P. Indanones as high-potency reversible inhibitors of monoamine oxidase. Chem. Med. Chem. 2015, 10, 862–873. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).