2-{[4-(4-Bromophenyl)piperazin-1-yl)]methyl}-4-(3-chlorophenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
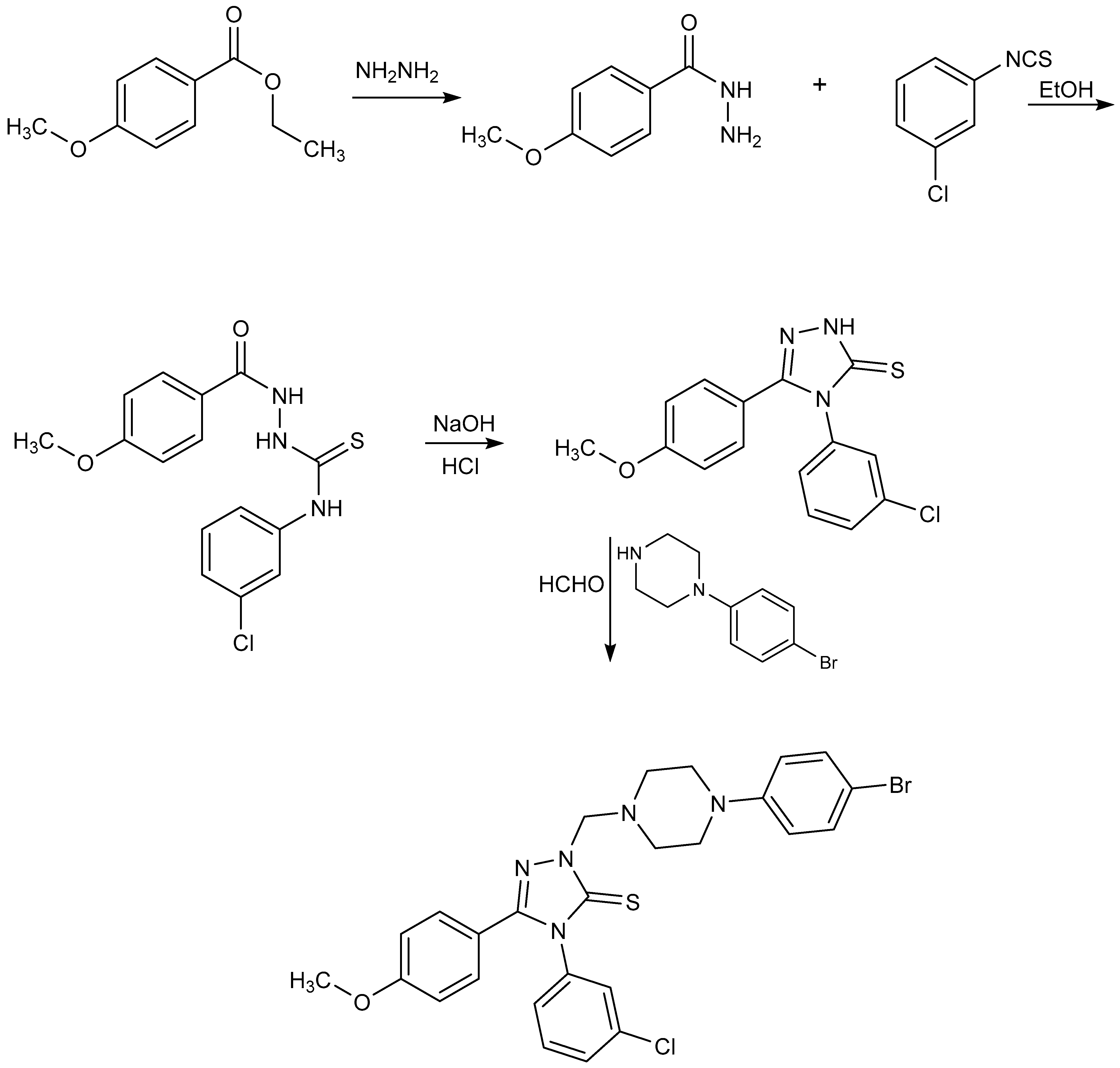
3.2. Synthesis of 2-{[4-(4-Bromophenyl)piperazin-1-yl]methyl}-4-(3-chlorophenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione
3.2.1. Synthesis of 4-Methoxybenzhydrazide [35]
3.2.2. Synthesis of 4-(3-Chlorophenyl)-1-(4-methoxyphenyl)thiosemicarbazide [35]
3.2.3. Synthesis of 4-(3-Chlorophenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione [35]
3.2.4. Synthesis of 2-{[4-(4-Bromophenyl)piperazin-1-yl]methyl}-4-(3-chlorophenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gul, H.I.; Tugrak, M.; Gul, M.; Mazlumoglu, S.; Sakagami, H.; Gulcin, I.; Supuran, T. New phenolic Mannich bases with piperazines and their bioactivities. Bioorg. Chem. 2019, 90, 103057. [Google Scholar] [CrossRef]
- Hafeez, F.; Zahoor, A.F.; Rasul, A.; Ahmad, S.; Mansha, A. Synthesis and anticancer evaluation of 2-oxo-2-(arylamino)-ethyl 4- phenylpiperazine-1-carbodithioates. Pak. J. Pharm. Sci. 2021, 34, 353–357. [Google Scholar] [PubMed]
- Al-Soud, Y.A.; Alhelal, K.A.S.; Saeed, B.A.; Abu-Qatouseh, L.; Al-Suod, H.H.; Al-Ahmad, H.; Al-Masoudi, N.A.; Al-Qawasmeh, R.A. Synthesis, anticancer activity and molecular docking studies of new 4-nitroimidazole derivatives. Arkivoc 2021, 8, 296–309. [Google Scholar] [CrossRef]
- Zárate, A.M.; Espinosa-Bustos, C.; Guerrero, S.; Fierro, A.; Oyarzún-Ampuero, F.; Quest, A.F.G.; Di Marcotullio, L.; Loricchio, E.; Caimano, M.; Calcaterra, A.; et al. A New Smoothened Antagonist Bearing the Purine Scaffold Shows Antitumour Activity In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 8372. [Google Scholar] [CrossRef] [PubMed]
- Abba, C.; Puram, N.; Betala, S. Synthesis of Novel Amide Functionalized Pyrido[2,3-d]pyrimidine Derivatives and their Anticancer Activity. Asian J. Chem. 2021, 33, 1579–1584. [Google Scholar] [CrossRef]
- Alagöz, M.A.; Özdemir, Z.; Uysal, M.; Carradori, S.; Gallorini, M.; Ricci, A.; Zara, S.; Mathew, B. Synthesis, Cytotoxicity and Anti-Proliferative Activity against AGS Cells of New 3(2H)-Pyridazinone Derivatives Endowed with a Piperazinyl Linker. Pharmaceuticals 2021, 14, 183. [Google Scholar] [CrossRef]
- Kaczor, A.; Szemerédi, N.; Kucwaj-Brysz, K.; Dąbrowska, M.; Starek, M.; Latacz, G.; Spengler, G.; Handzlik, J. Computer-Aided Search for 5-Arylideneimidazolone Anticancer Agents Able to Overcome ABCB1-Based Multidrug Resistance. ChemMedChem 2021, 16, 2386–2401. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Wang, X.; Zhang, Z. Synthesis, in vitro cytotoxicity and biological evaluation of twenty novel 1,3-benzenedisulfonyl piperazines as antiplatelet agents. Bioorg. Med. Chem. 2021, 46, 116390. [Google Scholar] [CrossRef]
- Sowmithri, S.; Kumar, J.V.S.; Mahesh, P.; Krishnamohan, T. Design and Synthesis of Novel Piperazine (2-Chloroethyl)-1-nitrosourea Analogues as Anticancer Agents. Asian J. Chem. 2022, 34, 591–596. [Google Scholar] [CrossRef]
- Baker, J.R.; Russel, C.C.; Gilbert, J.; McCluskey, A.; Sakoff, J.A. Amino alcohol acrylonitriles as broad spectrum and tumour selective cytotoxic agents. RSC Med. Chem. 2021, 12, 929–942. [Google Scholar] [CrossRef]
- Severin, A.O.; Pilyo, S.G.; Potikha, L.M.; Brovarets, V.S. Synthesis and Antitumor Activity of 5-Phenyl-1,3-thiazole-4-sulfonamide Derivatives. Russ. J. Gen. Chem. 2022, 92, 174–184. [Google Scholar] [CrossRef]
- Elgawish, M.S.; Nafie, M.S.; Yassen, A.S.A.; Yamada, K.; Ghareb, N. The design and synthesis of potent benzimidazole derivatives via scaffold hybridization and evaluating their antiproliferative and proapoptotic activity against breast and lung cancer cell lines. New J. Chem. 2022, 46, 4239. [Google Scholar] [CrossRef]
- Patel, S.; Globisch, C.; Pulugu, P.; Kumar, P.; Jain, A.; Shard, A. Novel imidazopyrimidines-based molecules induce tetramerization of tumor pyruvate kinase M2 and exhibit potent antiproliferative profile. Eur. J. Pharm. Sci. 2022, 170, 106112. [Google Scholar] [CrossRef]
- Song, J.; Liu, Y.; Yuan, X.Y.; Liu, W.B.; Li, Y.R.; Yu, G.X.; Tian, X.Y.; Zhang, Y.B.; Fu, X.J.; Zhang, S.Y. Discovery of 1,2,4-triazine dithiocarbamate derivatives as NEDDylation agonists to inhibit gastric cancers. Eur. J. Med. Chem. 2021, 225, 113801. [Google Scholar] [CrossRef]
- Rzycka-Korzec, R.; Malarz, K.; Gawecki, R.; Mrozek-Wilczkiewicz, A.; Małecki, J.G.; Schab-Balcerzak, E.; Korzec, M.; Polanski, J. Effect of the complex-formation ability of thiosemicarbazones containing (aza)benzene or 3-nitro-1,8-naphthalimide unit towards Cu(II) and Fe(III) ions on their anticancer activity. J. Photochem. Photobiol. A 2021, 415, 113314. [Google Scholar] [CrossRef]
- Silalai, P.; Pruksakorn, D.; Chairoungdua, A.; Suksen, K.; Saeeng, R. Synthesis of propargylamine mycophenolate analogues and their selective cytotoxic activity towards neuroblastoma SH-SY5Y cell line. Bioorg. Med. Chem. Lett. 2021, 45, 128135. [Google Scholar] [CrossRef]
- Desai, N.C.; Rupala, Y.M.; Khasiya, A.G.; Shah, K.N.; Pandit, U.P.; Khedkar, V.M. Synthesis, biological evaluation, and molecular docking study of thiophene-, piperazine-, and thiazolidinone-based hybrids as potential antimicrobial agents. J. Heterocyclic. Chem. 2022, 59, 75–87. [Google Scholar] [CrossRef]
- Baker, J.R.; Cossar, P.J.; Blaskovich, M.A.T.; Elliott, A.G.; Zuegg, J.; Cooper, M.A.; Lewis, P.J.; McCluskey, A. Amino Alcohols as Potential Antibiotic and Antifungal Leads. Molecules 2022, 27, 2050. [Google Scholar] [CrossRef]
- Foks, H.; Janowiec, M.; Zwolska, Z.; Augustynowicz-Kopeć, E. Synthesis and Tuberculostatic Activity of Some 2-Piperazinmethylene Derivatives 1,2,4-Triazole-3-Thiones. Phosphorus Sulfer Silicon 2005, 180, 537–543. [Google Scholar] [CrossRef]
- Sutherland, H.S.; Lu, G.; Tong, A.S.T.; Conole, D.; Franzblau, S.G.; Upton, A.M.; Lotlikar, M.U.; Cooper, C.B.; Palmer, B.D.; Choi, P.J.; et al. Synthesis and structure-activity relationships for a new class of tetrahydronaphthalene amide inhibitors of Mycobacterium tuberculosis. Eur. J. Chem. 2022, 229, 114059. [Google Scholar] [CrossRef]
- Patel, A.B.; Rohit, J.V. Development of 1,3,4-Thiadiazole and Piperazine Fused Hybrid Quinazoline Derivatives as Dynamic Antimycobacterial Agents. Polycycl. Aromat. Compd. 2022, 42, 5991–6002. [Google Scholar] [CrossRef]
- Varpe, B.D.; Jadhav, S.B. Schiff Base Conjugate of 5-Fluoroisatin with Thiophene-2-Ethylamine and its Mannich Bases: Synthesis, Molecular Docking, and Evaluation of in vitro Anti-inflammatory and Anti-tubercular Activity. Int. J. Pharm. Investig. 2021, 11, 189–194. [Google Scholar] [CrossRef]
- Gharbi, C.; Toumi, B.; Soudani, S.; Lefebvre, F.; Kaminsky, W.; Jelsch, C.; Nasr, C.B.; Khedhiri, L. Synthesis, structural characterization, antibacterial activity, DFT computational studies and thermal analysis of two new thiocyanate compounds based on 1-phenylpiperazine. J. Mol. Struct. 2022, 1257, 132620. [Google Scholar] [CrossRef]
- Plescia, C.B.; Lindstrom, A.R.; Quintero, M.V.; Keiser, P.; Anantpadma, M.; Davey, R.; Stahelin, R.V.; Davisson, V.J. Evaluation of Phenol-Substituted Diphyllin Derivatives as Selective Antagonists for Ebola Virus Entry. ACS Infect. Dis. 2022, 8, 942–957. [Google Scholar] [CrossRef]
- Mishra, S.; Parmar, N.; Chandrakar, P.; Sharma, C.P.; Parveen, S.; Vats, R.P.; Seth, A.; Goel, A.; Kar, S. Design, synthesis, in vitro and in vivo biological evaluation of pyranone-piperazine analogs as potent antileishmanial agents. Eur. J. Med. Chem. 2021, 221, 113516. [Google Scholar] [CrossRef]
- Kucwaj-Brysz, K.; Dela, A.; Podlewska, S.; Bednarski, M.; Siwek, A.; Satała, G.; Czarnota, K.; Handzlik, J.; Kiec-Kononowicz, K. The Structural Determinants for α1-Adrenergic/ Serotonin Receptors Activity aong PhenylpiperazineHydantoin Derivatives. Molecules 2021, 26, 7025. [Google Scholar] [CrossRef]
- Ji, L.; Fang, Y.; Tang, J.; Liu, C.; Huang, C.; Hu, Q.; Li, Q.; Chen, Z. Synthesis and biological evaluation of 18F-labelled dopamine D3 receptor selective ligands. Bioorg. Med. Chem. 2022, 62, 128630. [Google Scholar] [CrossRef]
- Lee, B.; Taylor, M.; Griffin, S.A.; McInnis, T.; Sumien, N.; Mach, R.H.; Luedtke, R.R. Evaluation of Substituted N-Phenylpiperazine Analogs as D3 vs. D2 Dopamine Receptor Subtype Selective Ligands. Molecules 2021, 26, 3182. [Google Scholar] [CrossRef]
- Waly, O.M.; Saad, K.M.; El-Subbagh, H.I.; Bayomi, S.M.; Ghaly, M.A. Synthesis, biological evaluation, and molecular modeling simulations of new heterocyclic hybrids as multi-targeted anti-Alzheimer’s agents. Eur. J. Med. Chem. 2022, 231, 114152. [Google Scholar] [CrossRef]
- Mohammadi-Khanaposthani, M.; Nori, M.; Valizadeh, Y.; Javanshir, S.; Dastyafteh, N.; Moaazam, A.; Hosseini, S.; Larijani, B.; Adibi, H.; Biglar, M.; et al. New 4-phenylpiperazine-carbodithioate-N-phenylacetamide hybrids: Synthesis, in vitro and in silico evaluations against cholinesterase and α-glucosidase enzymes. Arch. Pharm. 2022, 355, 2100313. [Google Scholar] [CrossRef]
- Ansari, S.; Noori, M.; Pedrood, K.; Mohammadi-Khanaposhtani, M.; Moazzam, A.; Hosseini, S.; Larijani, B.; Adibi, H.; Biglar, M.; Hamedifar, H.; et al. Novel aryl(4-phenylpiperazin-1-yl)methanethione derivatives as new anti-Alzheimer agents: Design, synthesis, in vitro and in silico assays. J. Mol. Struct. 2022, 1262, 132945. [Google Scholar] [CrossRef]
- Kumar, R.R.; Sahu, B.; Pathania, S.; Singh, P.K.; Akhtar, M.J.; Kumar, B. Piperazine, a Key Substructure for Antidepressants: Its Role in Developments and Structure-Activity Relationships. ChemMedChem 2021, 16, 1878–1901. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Czerwińska, K.; Madura, I.D.; Matuszewska, A.; Sporzyński, A.; Żubrowska-Zembrzuska, A. Piperazine derivatives of boronic acids—Potential bifunctional biologically active compounds. New J. Chem. 2015, 39, 4308–4315. [Google Scholar] [CrossRef]
- Paneth, A.; Trotsko, N.; Popiolek, Ł.; Grzegorczyk, A.; Krzanowski, T.; Janowska, S.; Malm, A.; Wujec, M. Synthesis and Antibacterial Evaluation of Mannich Bases Derived from 1,2,4-Triazole. Chem. Biodivers. 2019, 16, e1900377. [Google Scholar] [CrossRef] [PubMed]
- Swarnagowri, N.; Santosh, G.L.; Sushruta, S.H.; Swapna, B.; Nitinkumar, S.S. Synthesis, molecular docking and evaluation of library of 3-mercapto-1,2,4-triazole derivatives as antimicrobial agents. Asian J. Chem. 2021, 33, 3039–3046. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wujec, M.; Typek, R. 2-{[4-(4-Bromophenyl)piperazin-1-yl)]methyl}-4-(3-chlorophenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione. Molbank 2023, 2023, M1548. https://doi.org/10.3390/M1548
Wujec M, Typek R. 2-{[4-(4-Bromophenyl)piperazin-1-yl)]methyl}-4-(3-chlorophenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione. Molbank. 2023; 2023(1):M1548. https://doi.org/10.3390/M1548
Chicago/Turabian StyleWujec, Monika, and Rafał Typek. 2023. "2-{[4-(4-Bromophenyl)piperazin-1-yl)]methyl}-4-(3-chlorophenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione" Molbank 2023, no. 1: M1548. https://doi.org/10.3390/M1548
APA StyleWujec, M., & Typek, R. (2023). 2-{[4-(4-Bromophenyl)piperazin-1-yl)]methyl}-4-(3-chlorophenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione. Molbank, 2023(1), M1548. https://doi.org/10.3390/M1548






