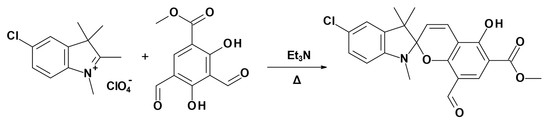
Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate
Abstract
1. Introduction
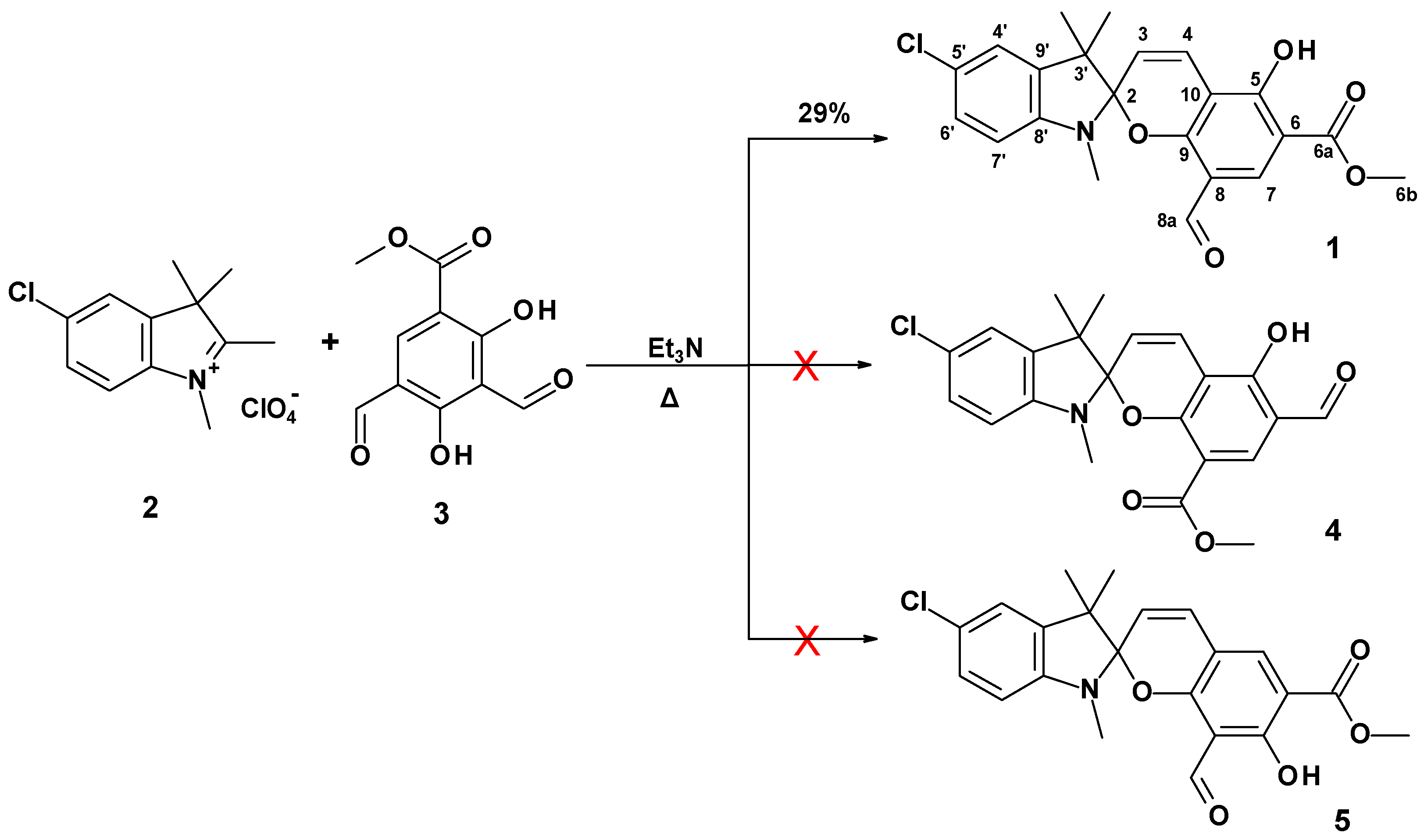
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Feringa, B.L.; Browne, W.R. Molecular Switches; Wiley-VCH: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bouas-Laurent, H.; Dürr, H. Organic photochromism (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 639–665. [Google Scholar] [CrossRef]
- Berman, E.; Fox, R.E.; Thomson, F.D. Photochromic Spiropyrans. I. The Effect of Substituents on the Rate of Ring Closure. J. Am. Chem. Soc. 1959, 81, 5605–5608. [Google Scholar] [CrossRef]
- Rad, J.K.; Balzade, Z.; Mahdavian, A.R. Spiropyran-based advanced photoswitchable materials: A fascinating pathway to the future stimuli-responsive devices. J. Photochem. Photobiol. C 2022, 51, 100487. [Google Scholar]
- Towns, A. Spiropyran dyes. Phys. Sci. Rev. 2021, 6, 20200197. [Google Scholar] [CrossRef]
- Kozlenko, A.S.; Pugachev, A.D.; Ozhogin, I.V.; El-Sewify, I.M.; Lukyanov, B.S. Spiropyrans: Molecules in motion. Chem. Heterocycl. Compd. 2021, 57, 984–989. [Google Scholar] [CrossRef]
- Kortekaas, L.; Browne, W.R. The evolution of spiropyran: Fundamentals and progress of an extraordinarily versatile photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef] [PubMed]
- Pugachev, A.D.; Ozhogin, I.V.; Makarova, N.I.; Rostovtseva, I.A.; Lukyanova, M.B.; Kozlenko, A.S.; Borodkin, G.S.; Tkachev, V.V.; El-Sewify, I.M.; Dorogan, I.V.; et al. Novel polychromogenic fluorine-substituted spiropyrans demonstrating either uni- or bidirectional photochromism as multipurpose molecular switches. Dye. Pigm. 2022, 199, 110043. [Google Scholar] [CrossRef]
- Gao, M.; Lian, C.; Xing, R.; Wang, J.; Wang, X.; Tian, Z. The photo-/thermo-chromism of spiropyran in alkanes as a temperature abuse indicator in the cold chain of vaccines. New J. Chem. 2020, 44, 15350–15353. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Mukhanov, E.L.; Ozhogin, I.V.; Kozlenko, A.S.; Metelitsa, A.V.; Lukyanov, B.S. Isomerization and changes of the properties of spiropyrans by mechanical stress: Advances and outlook. Chem. Heterocycl. Compd. 2021, 57, 122–130. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, H.; Zhang, G.; Zhou, Y.; Fan, L.; Shi, L.; Zhang, C.; Shuang, S.; Dong, C. Substituent effect on the acid-induced isomerization of spiropyran compounds. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 202, 13–17. [Google Scholar] [CrossRef]
- Ali, A.A.; Kharbash, R.; Kim, Y. Chemo- and biosensing applications of spiropyran and its derivatives—A review. Anal. Chim. Acta 2020, 1110, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Suda, M.; Kato, R.; Yamamoto, H.M. Light-induced superconductivity using a photoactive electric double layer. Science 2015, 347, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Minode, K.; Numai, Y.; Ohto, T.; Yamada, R.; Masai, H.; Tada, H.; Terao, J. Mechanical switching of current–voltage characteristics in spiropyran single-molecule junctions. Nanoscale 2020, 12, 7527–7531. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Sahoo, P.R.; Kumar, S. A MC-spiropyran for smartphone assisted reversible, selective and nanomolar level detection of formic acid in water and gas phase. J. Mol. Struct. 2021, 1223, 129249. [Google Scholar] [CrossRef]
- Ding, G.; Gai, F.; Gou, Z.; Zuo, Y. Multistimuli-responsive fluorescent probes based on spiropyrans for the visualization of lysosomal autophagy and anticounterfeiting. J. Mater. Chem. B 2022, 10, 4999–5007. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, X.; Zhu, S.; Liu, L.; Li, L. Spiropyran-Functionalized Gold Nanoclusters with Photochromic Ability for Light-Controlled Fluorescence Bioimaging. ACS Appl. Bio Mater. 2021, 4, 2790−2797. [Google Scholar] [CrossRef]
- Cardano, F.; Del Canto, E.; Giordani, S. Spiropyran for light-controlled drug delivery. Dalton Trans. 2019, 48, 15537–15544. [Google Scholar] [CrossRef]
- Barman, S.; Das, J.; Biswas, S.; Maitib, T.K.; Pradeep Singh, N.D. A spiropyran–coumarin platform: An environment sensitive photoresponsive drug delivery system for efficient cancer therapy. J. Mater. Chem. B 2017, 5, 3940–3944. [Google Scholar] [CrossRef]
- Lerch, M.M.; Hansen, M.J.; van Dam, G.M.; Szymanski, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016, 55, 10978. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Zolotukhin, P.V.; Mukhanov, E.L.; Rostovtseva, I.A.; Makarova, N.I.; Tkachev, V.V.; Beseda, D.K.; Metelitsa, A.V.; Lukyanov, B.S. Novel molecular hybrids of indolinespiropyrans and α-lipoic acid as potential photopharmacological agents: Synthesis, structure, photochromic and biological properties. Bioorg. Med. Chem. Lett. 2021, 31, 127709. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Tkachev, V.V.; Lukyanov, B.S.; Mukhanov, E.L.; Rostovtseva, I.A.; Lukyanova, M.B.; Shilov, G.V.; Strekal, N.D.; Aldoshin, S.M.; Minkin, V.I. Synthesis, structure and photochromic properties of novel highly functionalized spiropyrans of 1,3-benzoxazin-4-one series. J. Mol. Struc. 2018, 1161, 18–25. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Pugachev, A.D.; Tkachev, V.V.; Kozlenko, A.S.; Chepurnoi, P.B.; Dmitriev, V.S.; Shilov, G.V.; Aldoshin, S.M.; Minkin, V.I.; Lukyanov, B.S. Synthesis and study of interconversions of new indoline spiropyrans based on 4-hydroxy-3,5-diformylbenzoic acid. Russ. Chem. Bull. 2022, 71, 1710–1719. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Chernyavina, V.V.; Lukyanov, B.S.; Malay, V.I.; Rostovtseva, I.A.; Makarova, N.I.; Tkachev, V.V.; Lukyanova, M.B.; Metelitsa, A.V.; Aldoshin, S.M. Synthesis and study of new photochromic spiropyrans modified with carboxylic and aldehyde substituents. J. Mol. Struc. 2019, 1196, 409–416. [Google Scholar] [CrossRef]
- Parker, C.A.; Rees, W.T. Correction of fluorescence spectra and measurement of fluorescence quantum efficiency. Analyst 1960, 85, 587–600. [Google Scholar] [CrossRef]
- Magde, D.; Brannon, J.H.; Cremers, T.L.; Olmsted, J. Absolute luminescence yield of cresyl violet. A standard for the red. J. Phys. Chem. 1979, 83, 696–699. [Google Scholar] [CrossRef]
- Gel’man, N.E.; Terent’eva, E.A.; Shanina, T.M.; Kiparenko, L.M. Metody Kolichestvennogo Organicheskogo Elementnogo Analiza; Khimiya: Moscow, Russia, 1987. (In Russian) [Google Scholar]
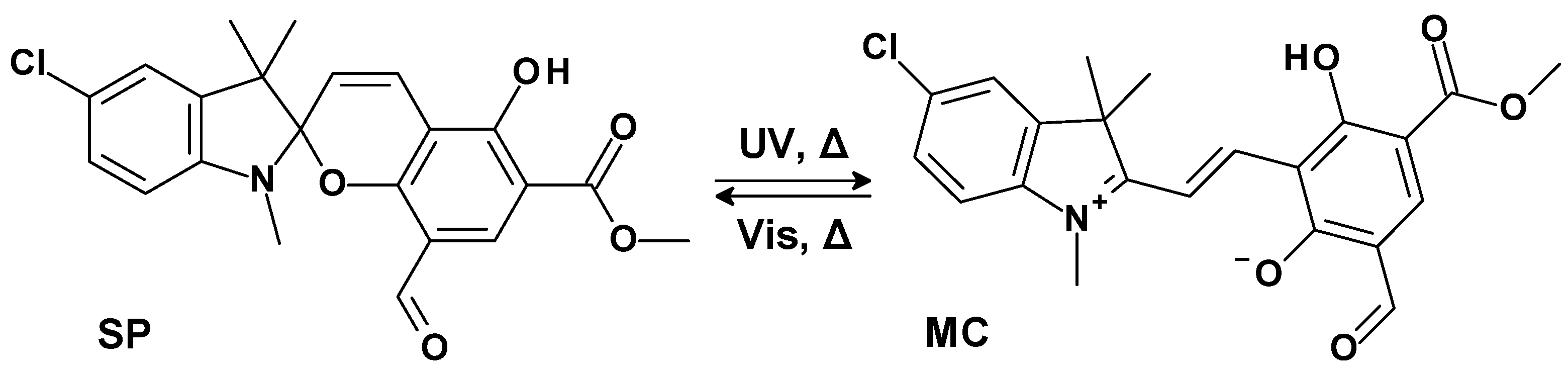
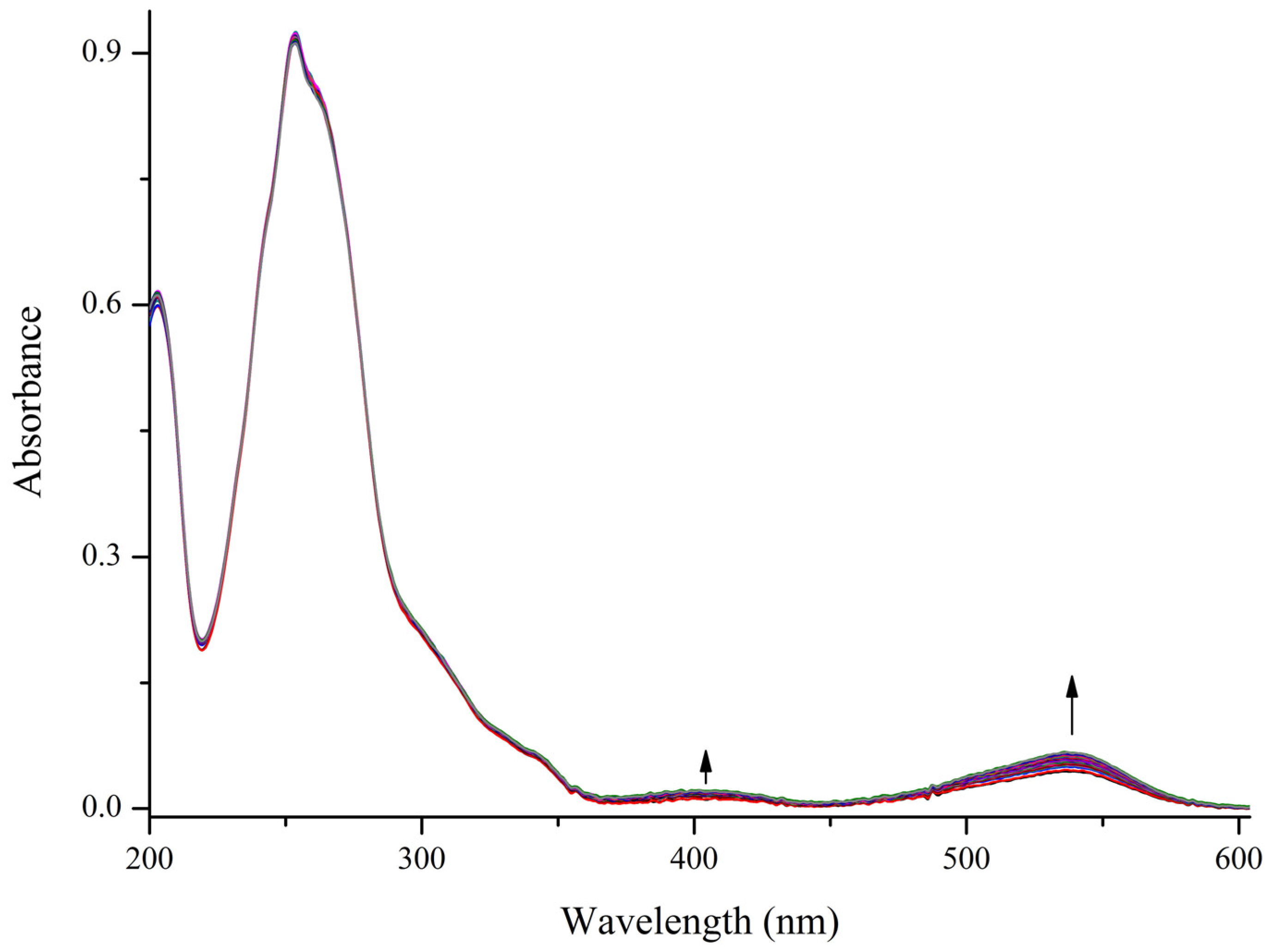
| Compound | Isomer | Absorption λmax, nm (ε·10−3, M−1·cm−1) | τ, s | Fluorescense λmax, nm (Φfl) |
|---|---|---|---|---|
| 1 | SP | 202 (33.4); 253 (47.1); 299 sh (11.2); 341 sh (3.5) | - | - |
| MC | 402; 536 | 105 | 611 (0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozhogin, I.V.; Pugachev, A.D.; Kozlenko, A.S.; Rostovtseva, I.A.; Makarova, N.I.; Borodkin, G.S.; El-Sewify, I.M.; Metelitsa, A.V.; Lukyanov, B.S. Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate. Molbank 2023, 2023, M1549. https://doi.org/10.3390/M1549
Ozhogin IV, Pugachev AD, Kozlenko AS, Rostovtseva IA, Makarova NI, Borodkin GS, El-Sewify IM, Metelitsa AV, Lukyanov BS. Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate. Molbank. 2023; 2023(1):M1549. https://doi.org/10.3390/M1549
Chicago/Turabian StyleOzhogin, Ilya V., Artem D. Pugachev, Anastasia S. Kozlenko, Irina A. Rostovtseva, Nadezhda I. Makarova, Gennady S. Borodkin, Islam M. El-Sewify, Anatoly V. Metelitsa, and Boris S. Lukyanov. 2023. "Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate" Molbank 2023, no. 1: M1549. https://doi.org/10.3390/M1549
APA StyleOzhogin, I. V., Pugachev, A. D., Kozlenko, A. S., Rostovtseva, I. A., Makarova, N. I., Borodkin, G. S., El-Sewify, I. M., Metelitsa, A. V., & Lukyanov, B. S. (2023). Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate. Molbank, 2023(1), M1549. https://doi.org/10.3390/M1549