1,1′-Dibromo-2,2′,5,5′-tetrakis(methylthio)ferrocene
Abstract
1. Introduction
2. Results
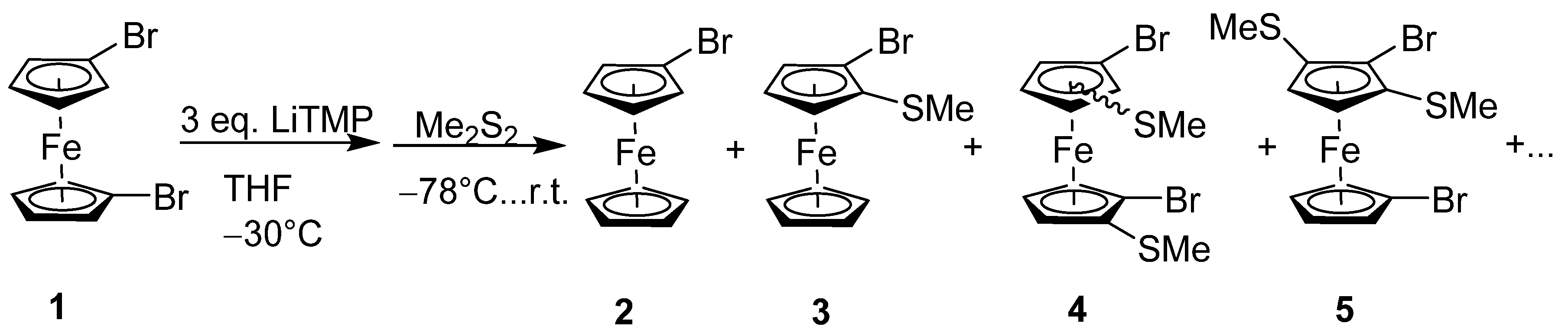
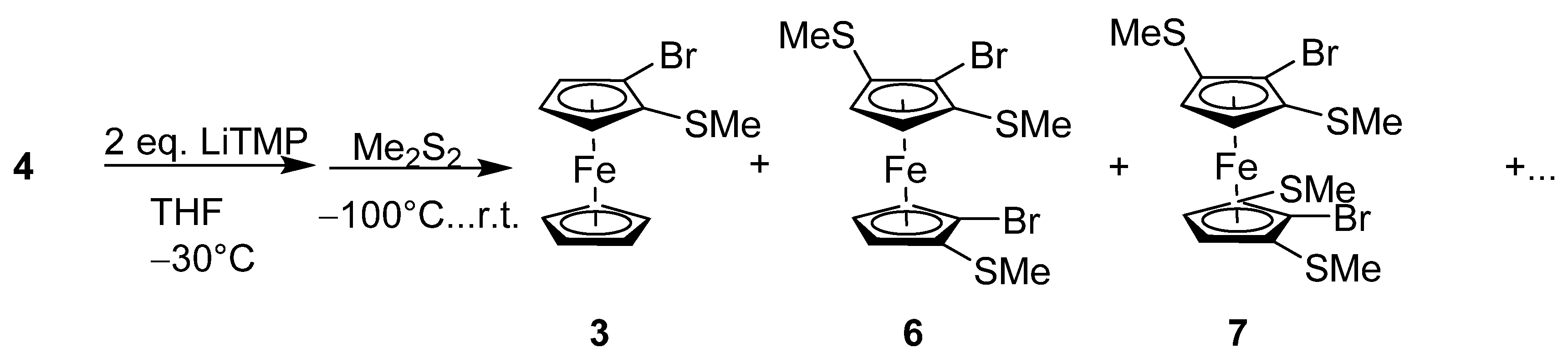
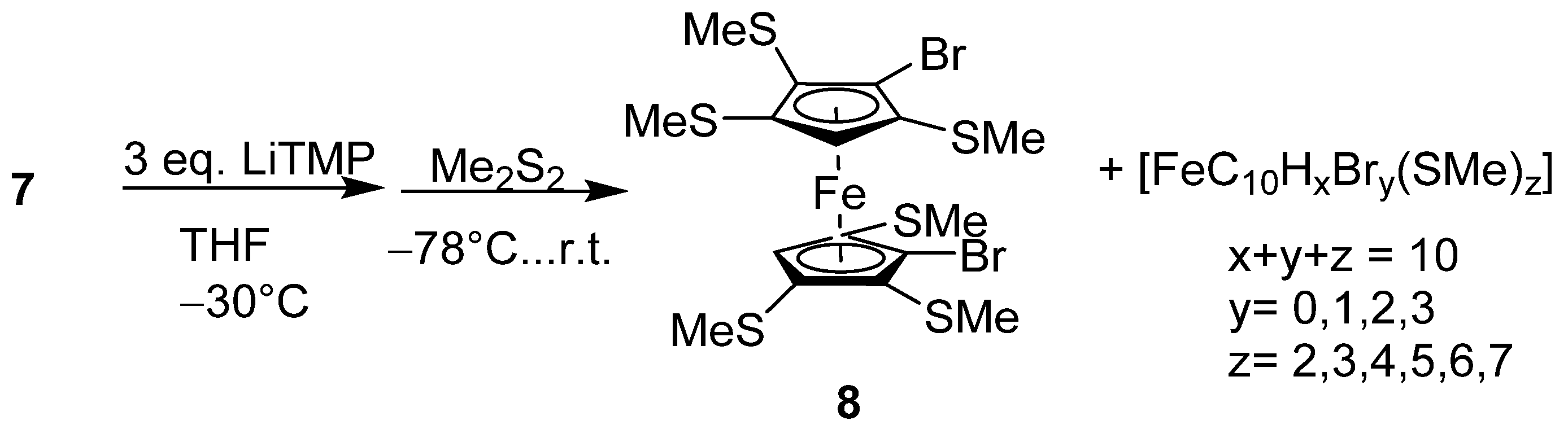
2.1. Synthesis and Reactivity
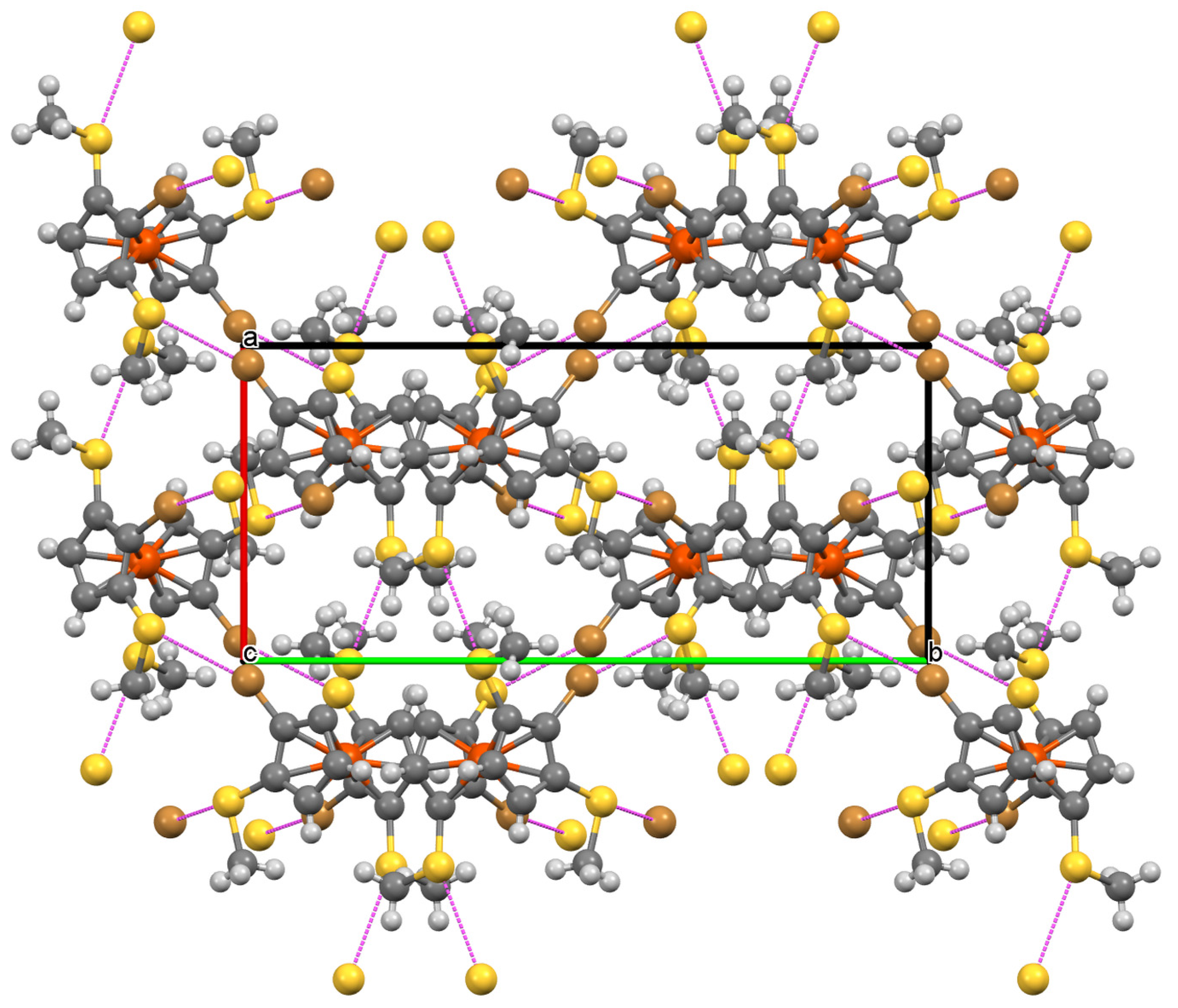
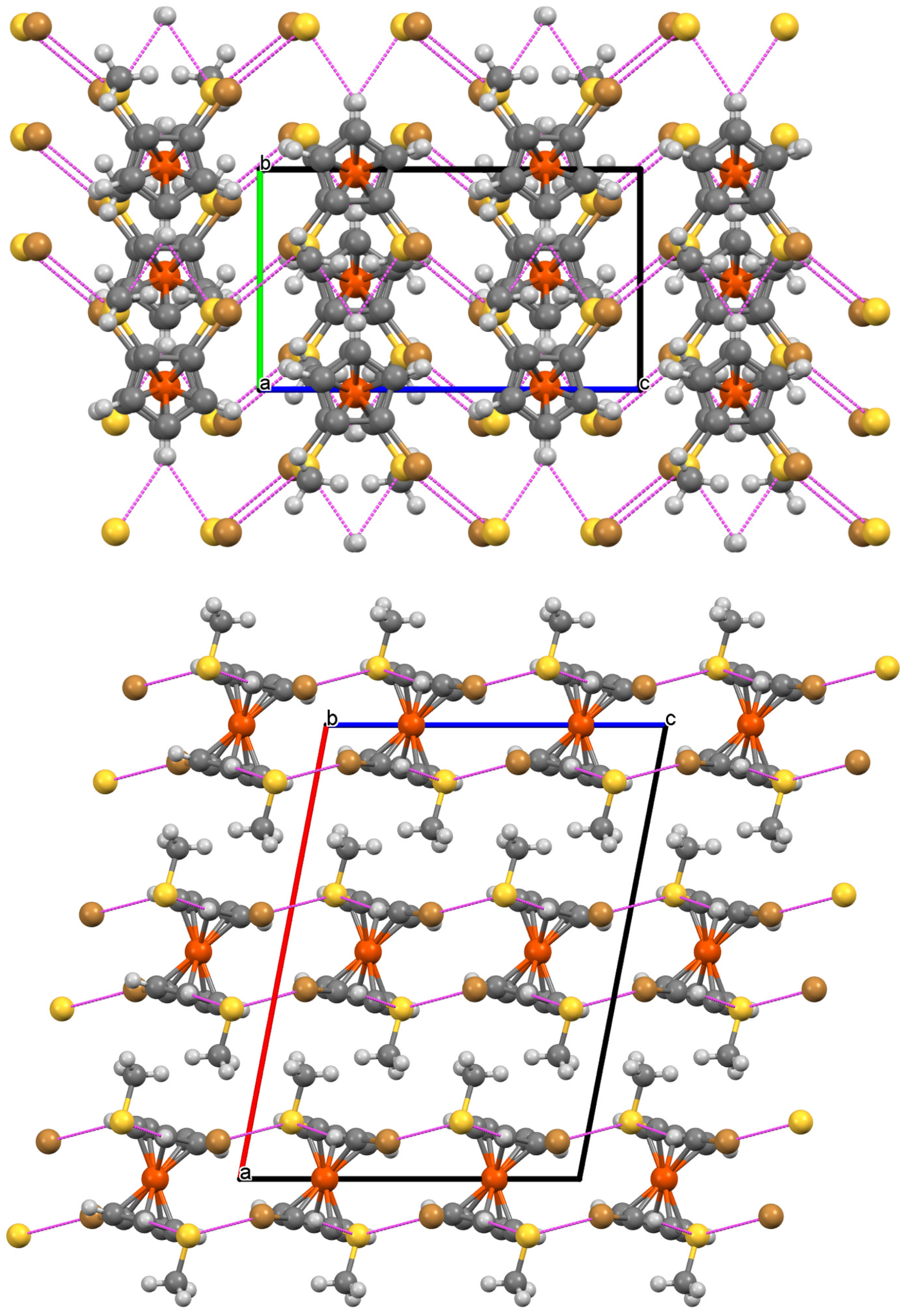
2.2. Molecular and Crystal Structure of 7
3. Discussion
4. Materials and Methods
4.1. Starting Materials, Reagents and Solvents
4.2. Reaction of 1,1′-Dibromoferrocene (1) with LiTMP and Me2S2
4.3. Reaction of 1,1′-Dibromo-2,2′-Bis(Methylthio)Ferrocene (4) with LiTMP and Me2S2
4.4. Reaction of 1,1′-Dibromo-2,5,2′-Tris(Methylthio)Ferrocene (6) with LiTMP and Me2S2
4.5. Reactivity Study with 1.1′-2,2′,5,5′-Tetrakismethylthioferrocene
4.6. Crystallography
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knox, G.R.; Pauson, P.L. Ferrocene Derivatives. Part VII. Some Sulphur Derivatives. J. Chem. Soc. 1958, 692–696. [Google Scholar] [CrossRef]
- Knox, G.R.; Pauson, P.L.; Tiers, G.V.D. A novel method for elucidation of structure by N.S.R. spectroscopy: Hindered rotation owing to adjacent substitution. Chem. Ind. 1959, 1046. [Google Scholar]
- Knox, G.R.; Morrison, I.G.; Pauson, P.L. Ferrocene Derivatives. Part XVI. The Aminomethylation of Methylthio-and Bismethylthio-ferrocene. J. Chem. Soc. (C) 1967, 1842–1847. [Google Scholar] [CrossRef]
- McCulloch, B.; Ward, D.L.; Woolins, D.; Brubaker, C.H., Jr. Ferrocenyl Sulfides. Preparation and Reactivity as Bidentate Chelating Ligands. Organometallics 1985, 4, 1425–1432. [Google Scholar] [CrossRef]
- Long, N.J.; Martin, J.; White, A.J.P.; Williams, D.J. Novel multidentate ferrocene ligands and their tungsten complexes. J. Chem. Soc. Dalton Trans. 1997, 26, 3083–3085. [Google Scholar] [CrossRef]
- Bushell, K.; Gialou, C.; Goh, C.H.; Long, N.J.; Martin, J.; White, A.J.P.; Williams, C.K.; Williams, D.J.; Fontani, M.; Zanello, P. Synthesis and characterization of novel multidentate ferrocene ligands and their Re(I) and Pt(II) complexes. J. Organomet. Chem. 2001, 637–639, 418–425. [Google Scholar] [CrossRef]
- Raghunath, M.; Gao, W.; Zhang, X. Ferrocenyl bis-phosphine ligands bearing sulfinylm sulfonyl or sulfenyl groups: Applications in asymmetric hydrogenation and allylic alkylation reactions. Tetrahedron Asymmetry 2005, 16, 3676–3681. [Google Scholar] [CrossRef]
- Butler, I.R.; Müssig, S.; Plath, M. A remarkably simple route to tri-substituted ferrocenes: The ortho-lithiation of 1,1′-dibromoferrocene and bromoferrocene. Inorg. Chem. Commun. 1999, 2, 424–427. [Google Scholar] [CrossRef]
- Butler, I.R.; Drew, M.G.B.; Greenwell, C.H.; Lewis, E.; Plath, M.; Mussig, S.; Szewczyk, J. 1,3-Bis-diphenylphosphinoferrocenes: The unexpected 2,5-dilithiation of dibromoferrocene towards a new area of ferrocene-ligand chemistry. Inorg. Chem. Commun. 1999, 2, 576–580. [Google Scholar] [CrossRef]
- Ilyashenko, G.; Al-Safadi, R.; Donnan, R.; Dubrovka, R.; Pancholi, J.; Watkinson, M.; Whiting, A. A synthesis of a 1,1′-desymmetrised ferrocene backbone and its facile one-pot double-click2 functionalisation. RSC Adv. 2013, 3, 17081–17087. [Google Scholar] [CrossRef]
- Bernhartzeder, S.; Kempinger, W.; Sünkel, K. Synthesis and reactivity of 1-chloro-2-methylthio-ferrocene and 1,1′-dichloro-2,2′-bis(methylthio)ferrocene. Molecular structure of 1-chloro-2,5-bis(methylthio)ferrocene. J. Organomet. Chem. 2014, 752, 147–151. [Google Scholar] [CrossRef]
- Blockhaus, T.; Klein-Hessling, C.; Zehetmaier, P.M.; Zott, F.L.; Jangra, H.; Karaghiosoff, K.; Sünkel, K. Ferrocenes with a Persulfurated Cyclopentadienyl Ring: Synthesis, Structural Studies, and Optoelectronic Properties. Chem. Eur. J. 2019, 25, 12655. [Google Scholar] [CrossRef]
- Butler, I.R.; Beaumont, M.; Bruce, M.I.; Zaitseva, N.N.; Iggo, J.A.; Robertson, C.; Horton, P.N.; Coles, S.J. 1, 1′,2,Tri-bromoferrocene, 1, 1′, 2,2′-Tetra-bromoferrocene, 1, 1′, 2,2′-Tetra-bromoruthenocene: Synthesis and Structure: Expanding the Range of Precursors for the Metallocene Chemist’s Toolkit. Aust. J. Chem. 2021, 74, 204–210. [Google Scholar] [CrossRef]
- Butler, I.R. The conversion of 1,1′-dibromoferrocene to 1,2-dibromoferrocene: The ferrocene-chemist’s dream reaction. Inorg. Chem. Commun. 2008, 11, 15–19. [Google Scholar] [CrossRef]
- Butler, I.R. Sitting out the Halogen Dance. The Room Temperature Formation of the 2,2′-Dilithio-1,1′-dibromoferrocene.TMEDA complex: A Synthetic Route to Multiply-substituted Ferrocenes. Organometallics 2021, 40, 3240–3244. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Blockhaus, T.; Bernhartzeder, S.; Kempinger, W.; Klein-Heßling, C.; Weigand, S.; Sünkel, K. Evidence for “Halogen Dance” and Ring-Exchange reactions in Chloro-methylthio-ferrocenes. Eur. J. Org. Chem. 2020, 2020, 6576–6587. [Google Scholar] [CrossRef]
- Inkpen, M.S.; Du, S.; Driver, M.; Albrecht, T.; Long, N.J. Oxidative purification of halogenated ferrocenes. Dalton Trans. 2013, 42, 2813–2816. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL- integrated space group and crystal structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Farrugia, L.J. WINGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
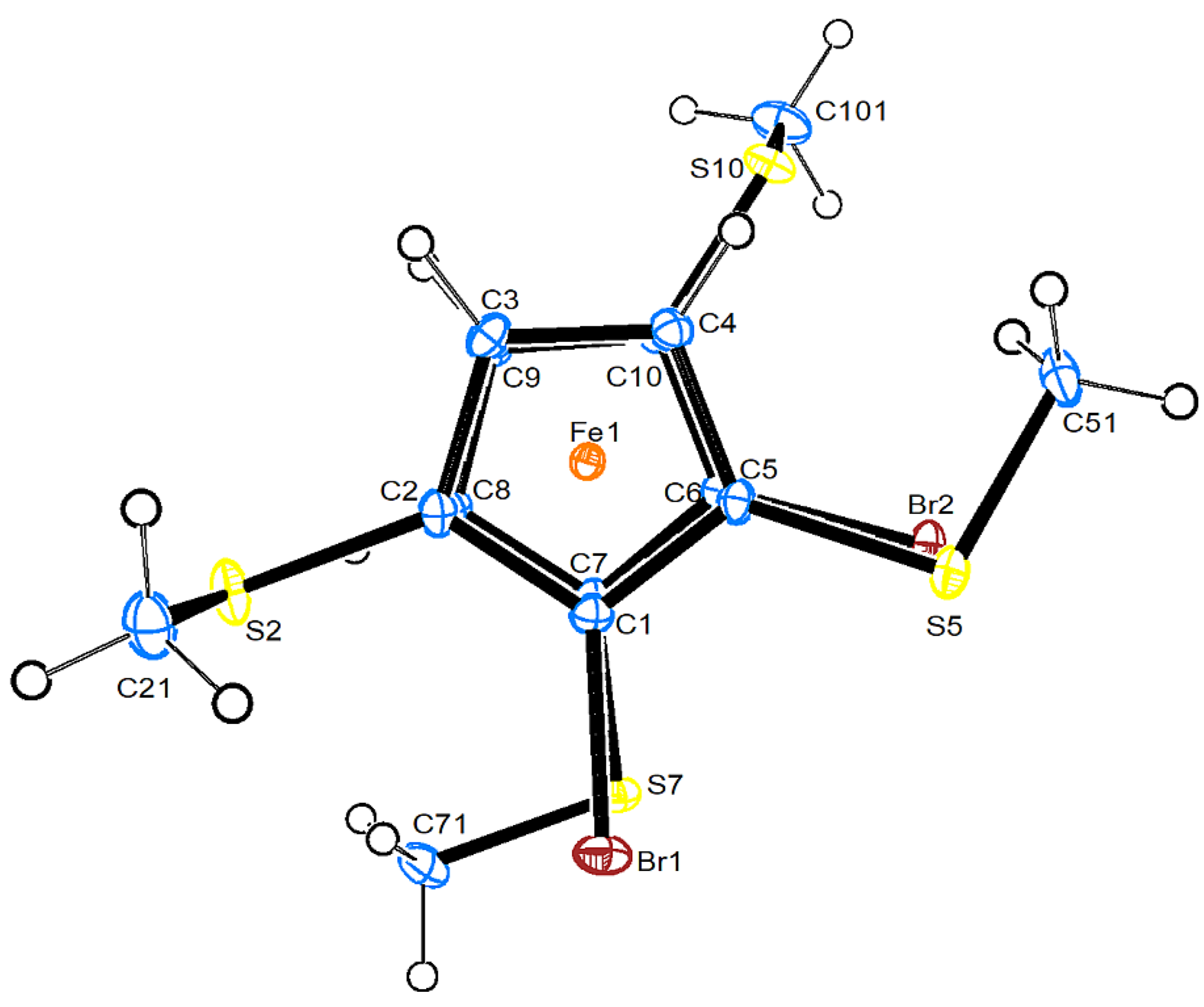
| Fe1–Ccp1 | 2.021(2) – 2.087(2) | Fe1–Ccp2 | 2.023(2)– 2.084(2) |
| Fe1–CT1 | 1.647(1) | Fe1–CT2 | 1.645(1) |
| Fe1…S2 | 3.3176(6) | Fe1…S10 | 3.3125(6) |
| C1–Br1 | 1.875(2) | C6–Br2 | 1.874 |
| C2–S2 | 1.754(2) | C7–S7 | 1.751(2) |
| C5–S5 | 1.746(2) | C10–S10 | 1.753(2) |
| Br1…CP1 | 0.089(1) | Br2…CP2 | 0.114(1) |
| S2…CP1 | −0.078(1) | S10…CP2 | −0.089(1) |
| S5…CP1 | 0.118(1) | C7…CP2 | 0.116(1) |
| C2-S2-C21 | 100.4(1) | C10-S10-C101 | 101.0(1) |
| C5-S5-C51 | 100.8(1) | C7-S7-C71 | 101.1(1) |
| CT1-Fe1_CT2 | 177.35(4) | ||
| C1-C2-S2-C21 | 90.7(2) | C6-C10-S10-C101 | 94.3(2) |
| C4-C5-S5-C51 | −16.4(2) | C8-C7-S7-C71 | −12.9(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blockhaus, T.; Zott, F.; Sünkel, K. 1,1′-Dibromo-2,2′,5,5′-tetrakis(methylthio)ferrocene. Molbank 2023, 2023, M1542. https://doi.org/10.3390/M1542
Blockhaus T, Zott F, Sünkel K. 1,1′-Dibromo-2,2′,5,5′-tetrakis(methylthio)ferrocene. Molbank. 2023; 2023(1):M1542. https://doi.org/10.3390/M1542
Chicago/Turabian StyleBlockhaus, Tobias, Fabian Zott, and Karlheinz Sünkel. 2023. "1,1′-Dibromo-2,2′,5,5′-tetrakis(methylthio)ferrocene" Molbank 2023, no. 1: M1542. https://doi.org/10.3390/M1542
APA StyleBlockhaus, T., Zott, F., & Sünkel, K. (2023). 1,1′-Dibromo-2,2′,5,5′-tetrakis(methylthio)ferrocene. Molbank, 2023(1), M1542. https://doi.org/10.3390/M1542






