Abstract
In this paper, we report the crystal structure of 2-[2-(diphenylphosphoryl)phenyl]-1H-perimidine (L1) obtained from the ring closure reaction of 1,8-diaminonaphthalene and 2-(diphenylphosphino)benzaldehyde, followed by the dehydrogenation reaction with sodium metabisulfite (Na2S2O5). L1 was characterised using 1H, 13C & 31P NMR, FT-IR and X-ray single structure analyses.
1. Introduction
N-heterocyclic moieties with substantial biological properties are becoming diverse and popular with researchers. There are a number of N-heterocyclic compounds that are broadly dispersed in nature; possess physiological and pharmacological properties; and are constituents of numerous biologically important molecules, such as vitamins, nucleic acids, antibiotics and pharmaceuticals [1,2,3,4]. The nitrogen heterocycle is electron-rich; thus, it is able to accept or donate a proton, and it can easily establish diverse weak interactions. An example of such is the perimidine ligand, which has a rich chemistry due to the existence of a lone pair of nitrogen atoms capable of transferring their electron density to the naphthalene ring from the fused heterocyclic ring [5]. Perimidines are versatile ligand frameworks and are an attractive class of N-heterocycles that have different applications in medical sciences, industrial chemistry and life sciences [5]. The interest in perimidine ligands stems from their numerous biological activities as ligands [6,7,8,9,10] and when coordinated with different transition metals, such as ruthenium, cobalt, nickel, copper, zinc, vanadium and palladium [11,12,13,14,15]. Due to the excellent electronic features and diverse applications in different areas, we are interested in the synthesis of mixed-donor atom ligands of perimidine and phosphorus. Phosphorus is a good electron donor, and it is easy to optimise its electronic and steric properties. Compounds that contain soft (P) and hard (N) Lewis bases are able to stabilise different oxidation states of metals, therefore generating complexes with different reactivities, applications and spectroscopic properties [16]. This paper reports the single-crystal structural data of a mixed-donor ligand, 2-[2-(diphenylphosphoryl)phenyl]-1H-perimidine, and its other characterization data collected using various spectral techniques.
2. Results
2-[2-(diphenylphosphoryl)phenyl]-1H-perimidine (L1) was obtained in moderate yield by the ring closure reaction of 1,8-diaminonaphthalene and 2-(diphenylphosphino)benzaldehyde in the presence of sodium metabisulfite in a solution of ethanol and water. The sodium metabisulfite was used to perform an oxidative dehydrogenation reaction following the condensation reaction of 1,8-diaminonaphthalene and 2-(diphenylphosphino)benzaldehyde. The formation of the compound was revealed by the absence of a singlet peak resonating at around 5.5 ppm in the 1H NMR spectrum of L1, which suggests that dehydrogenation occurred during the ring closure, therefore revealing a new C=N bond (Figure S1). The presence of a broad singlet peak at 9.39 ppm, which corresponds to one proton, assigned to the -NH group, clearly confirms the formation of a new bond, -NH-C. In the 13C NMR spectrum (Figure S2), the expected chemical shifts of L1 are observed, and the presence of a peak resonating at 154.14 ppm, which corresponds to the new bond between C=N and C-N, further confirms the formation of L1. In the FT-IR spectrum of L1 (Figure S4), the appearance of an absorption band at 1657 cm−1 assigned to the C=N group clearly indicates the successful ring closure reaction of the aldehyde and the diamine. The peak at 1311 cm−1 corresponds to the vibrational signals of the C-N single bond, which also confirms the presence of a new C-N single bond. The appearance of one stretching band at 3442 cm−1 confirms the presence of a secondary N-H group, which clearly indicates the presence of a new C-NH bond in the compound.
Single X-ray analyses of L1 grown via the slow diffusion of methanol solvent in dichloromethane solution at room temperature were carried out. Figure 1 shows the molecular structure of L1, while Table 1 (bond length and angles) contains selected geometrical parameters of L1. The solid-state structure of L1 adopts a monoclinic crystal structure and a P21/n space group. In the structure of the title compound, the bond angles N1-C11-N2, N1-C11-C12 and N2-C11-C12 are 125.63(12), 116.92(11) and 117.45(11)°, respectively, indicative of the sp2 hybridization state of the C(11) atom [17]. The torsional angle between the perimidine ring and the phenyl ring is 48.35°. The bond length of N2-C11 (1.3581(17) Å) and the bond length of N1-C11 (1.2948(17)) are both close to the average C-N bond length [13,18,19,20] reported. The packing structure of L1 (Figure 2) also indicates strong intermolecular hydrogen bonding between the N-H · · · · ·OP bonds, which can be clearly seen in Figure 3.
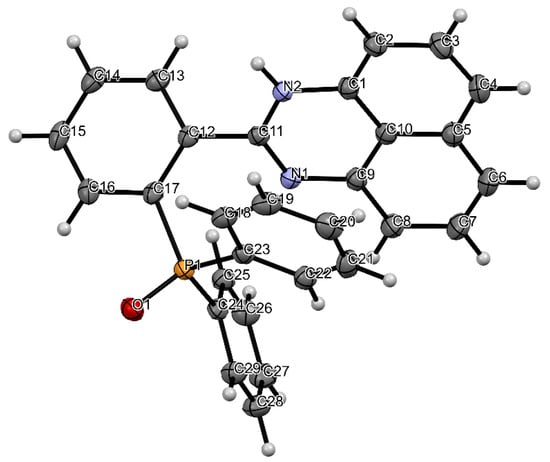
Figure 1.
ORTEP diagram of L1 with the thermal ellipsoids drawn at the 50% probability level. Atom colours: (a) blue = nitrogen, (b) white = hydrogen, (c) red = oxygen, (d) orange = phosphorus, (e) grey = carbon.

Table 1.
Selected bond length (Å) and angles (°) of L1.
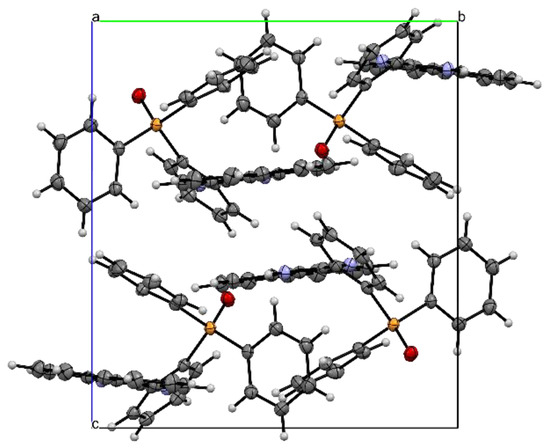
Figure 2.
Packing diagram of L1 along b axis. Atom colours: (a) blue = nitrogen, (b) white = hydrogen, (c) red = oxygen, (d) orange = phosphorus, (e) grey = carbon.
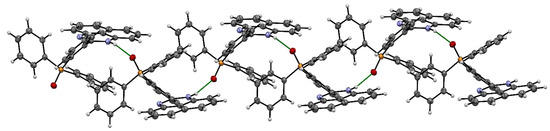
Figure 3.
A hydrogen-bonded structure of L1 formed via N-H · · · · ·O bonds. Atom colours: (a) blue = nitrogen, (b) white = hydrogen, (c) red = oxygen, (d) orange = phosphorus, (e) grey = carbon.
3. Materials and Methods
Ethanol 99.5%, dichloromethane 99.8%, 1,8-diaminonaphthalene 99%, 2-(diphenylphosphino)benzaldehyde 97%, sodium metabisulfite 99% and anhydrous magnesium sulphate 97% were purchased from Sigma-Aldrich. All chemicals were of analytical-grade and were used as supplied. The NMR spectra were recorded on a Bruker Avance III 400 MHz NMR spectroscope with a 5 mm TBIZ probe at 30 °C. Chemical shifts are reported in parts per million (ppm) in relation to the solvent (CDCl3) residual peak, 7.26 and 77.16 ppm for 1H and 13C NMR, respectively. The coupling constants (J) were calculated in Hertz (Hz), and the splitting pattern was designated as s for singlet, d for doublet, t for triplet and m for multiplet. The infrared spectrum was recorded using a Bruker Alpha II FT-IR spectrometer, and the data are reported as percentage transmittance at the respective wavenumbers (cm−1), between 4000 and 450 cm−1.
3.1. Synthesis of 2-[2-(Diphenylphosphoryl)phenyl]-1H-perimidine (L1)
L1 was prepared in moderate yield (Scheme 1) by the ring closure reaction of 1,8-diaminonaphthalene and 2-(diphenylphosphino)benzaldehyde. A mixture of ethanol and water (v:v = 5:1) was added to 1,8-diaminonaphthalene (0.501 mmol, 79.4 mg), 2-(diphenylphosphino)benzaldehyde (0.498 mmol, 144.6 mg) and Na2S2O5 (1.50 mmol, 285.17 mg) in a 100 mL round-bottomed flask. The reaction mixture was refluxed at 75 °C for 24 h and formed a precipitate. The resulting precipitate was filtered, washed with 10 mL of ethanol and 20 mL of water, then dissolved in dichloromethane and extracted with water. Magnesium sulphate was added to the solution and filtered. The solvent was removed under reduced pressure to form an orange solid. Yield: 95.2 mg (43.0%), 1H NMR (400 MHz, CDCl3): δ (ppm) 9.39 (s, 1H, NH), 8.64 (d, J = 4.88 Hz, 2H, Ar-H), 8.45 (d, J = 8.17 Hz, 2H, Ar-H), 7.89 (td, J = 7.61, 1.68 Hz, 2H, Ar-H), 7.46 (ddd, J = 12.35, 7.82, 6.20 Hz, 2H, Ar-H), 7.26 (d, J = 7.61 Hz, 2H, Ar-H), 7.09–7.16 (m, 6H, Ar-H), 6.92 (d, J = 7.33 Hz, 2H,), 6.37 (d, J = 7.04 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 154.1, 134.9, 133.6 (d, J = 11.4 Hz), 133.1 (d, J = 2 Hz), 132.5 (d, J = 3 Hz, 2 × 4-CH of PPh), 132.0 (d, J = 8.8 Hz), 131.6 (d, J = 10 Hz, 4 × 2-CH of PPh), 131.2, 130.5 (d, J = 12 Hz), 130.2, 129.1, 128.7 (d, J = 12.4 Hz, 4 × 3-CH of PPh), 128.1, 121.4, 119.9. 31P NMR (162 MHz, CDCl3): δ = 35.03 (s). FT-IR (cm−1): 3442 (NH), 2995 (CH), 2912 (CH), 1657 (C=N), 1420 (C=C), 1311 (C-N), 1031 (C-H), 946 (C-H), 692 (C-H), 525 (C-H).
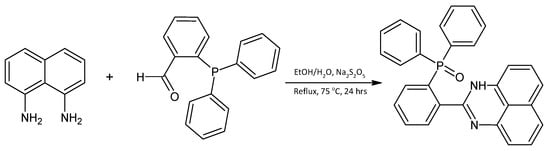
Scheme 1.
Synthesis of 2-[2-(diphenylphosphoryl)phenyl]-1H-perimidine (L1).
3.2. X-ray Crystallography
The X-ray crystallographic data of L1 were collected and evaluated on a Bruker APEX-II Duo [21] CCD area detector diffractometer with an Incoatec micro-source working at 30 W power. An Oxford Instruments Cryojet accessory was used to keep the crystal at 104.70 K during data collection. The data were collected with Cu(Kα), λ = 1.54178) at a crystal-to-detector distance of 50 mm. The data were reduced using the SAINT [22] program. The non-hydrogen atoms were initially refined isotropically, and then anisotropic refinement was carried out using a full-matrix least-squares method based on F2. Olex2 [23] was used to solve the crystal structure, while the SHELXT [24] and SHELXL [25] programs were used for structural refinement using intrinsic phasing and least-squares minimisation. The crystallographic data were visualized using WinGX [26] and Mercury v.4.3 [27]. The crystallographic data and structure refinement parameters of L1 are given in Table 2.

Table 2.
Crystal data and structure refinement of L1.
4. Conclusions
2-[2-(Diphenylphosphoryl)phenyl]-1H-perimidine (L1) was synthesised from the ring closure reaction of 1,8-diaminonaphthalene and 2-(diphenylphosphino)benzaldehyde, followed by the dehydrogenation reaction with sodium metabisulfite. The purity of L1 was confirmed using 1H, 13C and 31P NMR, FT-IR and LC-MS spectroscopic techniques and single X-ray crystallography. L1 crystallizes in the monoclinic crystal system and the P21/n space group, and it has strong intermolecular hydrogen bonding between the N-H · · · · ·OP bonds in its packing structure.
Supplementary Materials
The following supporting information can be downloaded. Figure S1: 1H NMR spectrum of 2-[2-(diphenylphosphoryl)phenyl]-1H-perimidine (L1). Figure S2: 13C NMR spectrum of 2-[2-(diphenylphosphoryl)phenyl]-1H-perimidine (L1). Figure S3: 31P NMR spectrum of 2-[2-(diphenylphosphoryl)phenyl]-1H-perimidine (L1). Figure S4: IR spectrum of 2-[2-(diphenylphosphoryl)phenyl]-1H-perimidine (L1).
Author Contributions
S.S., conceptualization, structure design, provision of synthesis materials and supervision. M.N.G., synthesis, crystal growth and characterization. T.R.P., supervision, formal analysis and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
CDCC No: 2221152 contains the supplementary crystallographic data for the title compound. The data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44)1223-336-033; or via email: deposit@ccdc.cam.ac.uk.
Acknowledgments
The authors are grateful to the University of KwaZulu-Natal for financial support and for providing resources for this research.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Ju, Y.; Varma, R.S. Aqueous N-heterocyclization of primary amines and hydrazines with dihalides: Microwave-assisted syntheses of N-azacycloalkanes, isoindole, pyrazole, pyrazolidine, and phthalazine derivatives. J. Org. Chem. 2006, 71, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari-Sis, B.; Zirak, M.; Akbari, A. Arylglyoxals in synthesis of heterocyclic compounds. Chem. Rev. 2013, 113, 2958–3043. [Google Scholar] [CrossRef] [PubMed]
- Zarate, D.Z.; Aguilar, R.; Hernandez-Benitez, R.I.; Labarrios, E.M.; Delgado, F.; Tamariz, J. Synthesis of α-ketols by functionalization of captodative alkenes and divergent preparation of heterocycles and natural products. Tetrahedron 2015, 71, 6961–6978. [Google Scholar] [CrossRef]
- Kerru, N.; Maddila, S.; Jonnalagadda, S.B. Design of carbon–carbon and carbon–heteroatom bond formation reactions under green conditions. Curr. Org. Chem. 2019, 23, 3156–3192. [Google Scholar] [CrossRef]
- Sahiba, N.; Agarwal, S. Recent advances in the synthesis of perimidines and their applications. Top. Curr. Chem. 2020, 378, 44–91. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gijon, C.A.; Olvera-Mancilla, J.; Lagadec, R.L.; Barba-Behrens, N.; Rico-Bautista, H.; Toscano, R.A.; Alexandrova, L. 2-Substituted perimidines: Zwitterionic tauterism in solid state, substituent effect on their crystal packing and biological activity. J. Mol. Stuct. 2022, 1252, 132056–132065. [Google Scholar] [CrossRef]
- Bassyouni, F.B.; Abu-Bakr, S.M.; Hegab, K.H.; El-Eraky, W.; ElBeih, A.A.; Abdel Rehim, M.E. Synthesis of new transition metal complexes of 1H-perimidine derivatives having antimicrobial and anti-inflammatory activities. Res. Chem. Intermed. 2012, 38, 1527–1550. [Google Scholar] [CrossRef]
- Nagasundaram, N.; Govindhan, C.; Sumitha, S.; Sedhu, N.; Raguvaran, K.; Santhosh, S.; Lalitha, A. Synthesis, characterization and biological evaluation of novel azo fused 2,3-dihydro-1H-perimidine derivatives: In vitro antibacterial, antibiofilm, anti-quorum sensing, DFT, in silico ADME and Molecular docking studies. J. Mol. Stuct. 2022, 1248, 131437–131459. [Google Scholar] [CrossRef]
- Menteşe, E.; Yilmaz, F.; Karaali, N.; Ülker, S.; Kahveci, B. Rapid synthesis and lipase inhibition activity of some new benzimidazole and perimidine derivatives. Russ. J. Bioorg. Chem. 2004, 40, 336–342. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, X.Z.; Cao, Q.; Gong, G.H.; Quan, Z.S. Design, synthesis, anti-inflammatory activity, and molecular docking studies of perimidine derivatives containing triazole. Bioorg. Med. Chem. Lett. 2017, 27, 4409–4414. [Google Scholar] [CrossRef]
- Azam, M.; Warad, I.; Al-Rasayes, S.; Zahin, M.; Ahmad, I.; Shakir, M. Syntheses, Physico-chemical studies and antioxidant activities of transition metal complexes with a perimidine ligand. Z. Anorg. Allg. Chem. 2012, 638, 881–886. [Google Scholar] [CrossRef]
- Azam, M.; Warad, I.; Al-Rasayes, S.; Alzaqri, N.; Khan, M.R.; Pallepogu, R.; Dwivedi, S.; Musarrat, J.; Shakir, M. Synthesis and structural characterization of Pd(II) complexes derived from perimidine ligand and their in vitro antimicrobial studies. J. Mol. Struct. 2013, 1047, 48–54. [Google Scholar] [CrossRef]
- Booysen, I.N.; Ebinumolishe, I.; Sithebe, S.; Akerman, M.P.; Xulu, B. Coordination behaviours of perimidine ligands incorporating fused N-donor heterocyclics towards rhenium(I) and -(V). Polyhedron 2016, 117, 755–7760. [Google Scholar] [CrossRef]
- Al-Hazmi, G.A.; Abou-Melha, K.S.; El-Metwaly, E.M.; Saleh, K.A. Synthesis of Novel VO(II)-Perimidine complexes: Spectral, computational, and antitumor studies. Bioinorg. Chem. And Appl. 2018, 2018, 7176040. [Google Scholar] [CrossRef]
- Patel, U.N.; Sungh, A. Metal complexation studies of 1-(4-Carboxy-3-hydroxy-N-methyl phenyl- amino methyl) 2-methyl perimidine. J. Chem. 2009, 6, S452–S458. [Google Scholar] [CrossRef]
- Munzeiwa, W.A.; Omondi, B.; Nyamori, V.O. Architecture and synthesis of P,N-heterocyclic phosphine ligands. Beilstein J. Org. Chem. 2020, 16, 362–383. [Google Scholar] [CrossRef]
- Yuan, L.; Li, Z.-Y.; Yuan, X.-Y.; Zhang, M.; Liu, F. Crystal structure of 1,3-dimethyl-2-phenyl-1H-perimidin-3-ium iodide, C19H17IN2. Z. Für Krist. New Cryst. Struct. 2017, 232, 541–543. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Yin, G.; Du, C.; Zhang, B. Efficiently luminescent heteroleptic neutral platinum(II) complexes based on N^O and N^P benzimidazole ligands. Dalton Trans. 2021, 50, 17319–17327. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Du, C.; Zhang, B. Efficiently red emitting cycloplatinated(II) complexes supported by N^O and N^P benzimidazole ancillary ligands. J. Organomet. Chem. 2022, 960, 122237–122246. [Google Scholar] [CrossRef]
- Llamas-Saiz, A.L.; Foces-Foces, C.; Sanz, D.; Claramunt, R.M.; Dotor, J.; Elguero, J.; Catalan, J.; del Valle, J.C. 2-Arylperimidine derivatives. Part 1. Synthesis, NMR spectroscopy, X-ray crystal and molecular structures. J. Chem. Soc. Perkin Trans. 1995, 2, 1389–1398. [Google Scholar] [CrossRef]
- CCD CrysAlis. CrysAlis Red; Xcalibur PX Software; Oxford Diffraction Ltd.: Abingdon, UK, 2008. [Google Scholar]
- Saint, B. Data Reduction Software, (v7.68A); Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Streek, J.; Wood, P.A. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).