N-(3-Chlorophenethyl)-2-(4-isobutylphenyl)propanamide
Abstract
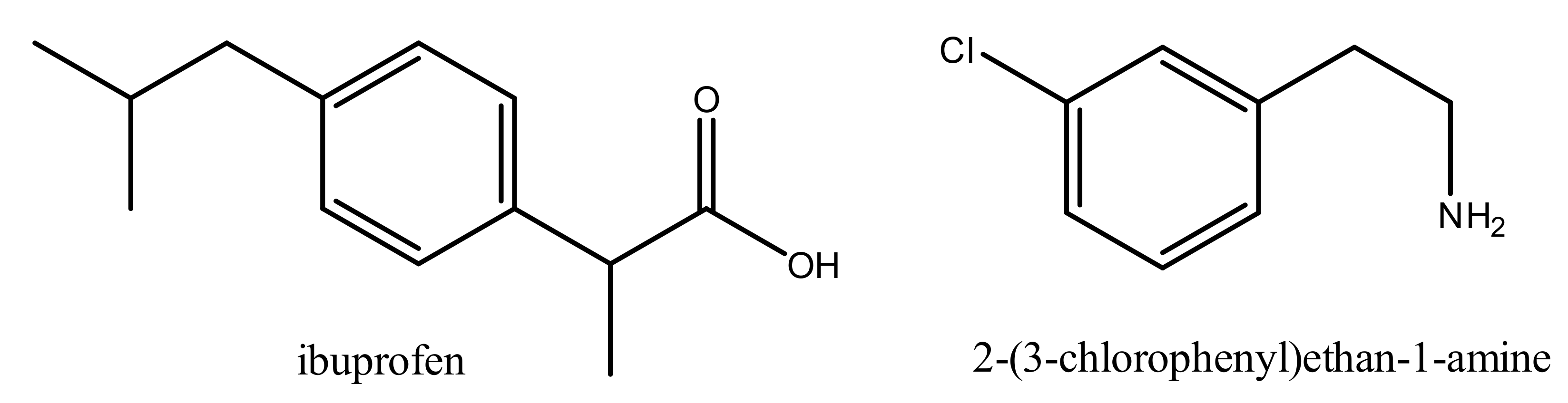
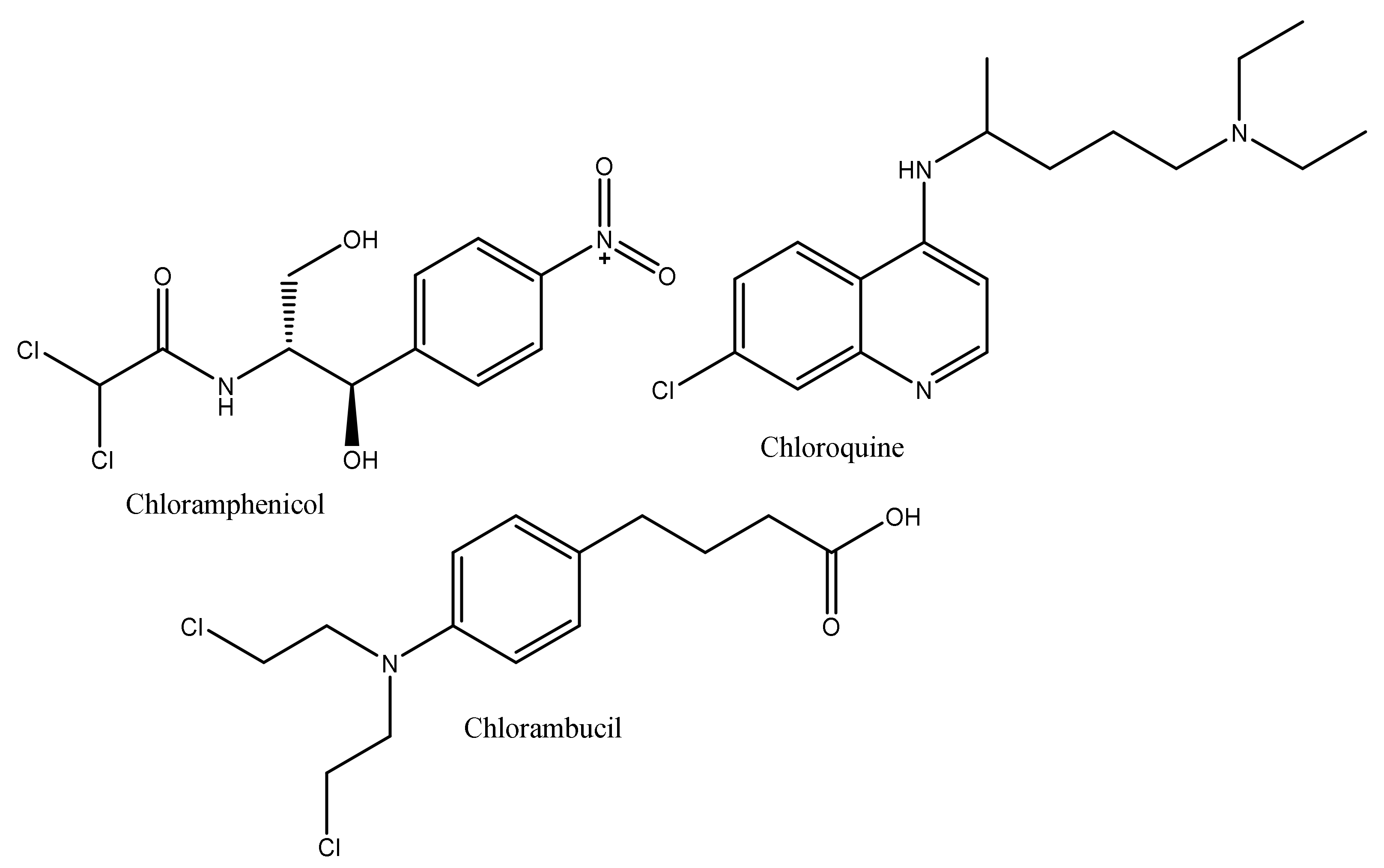
1. Introduction
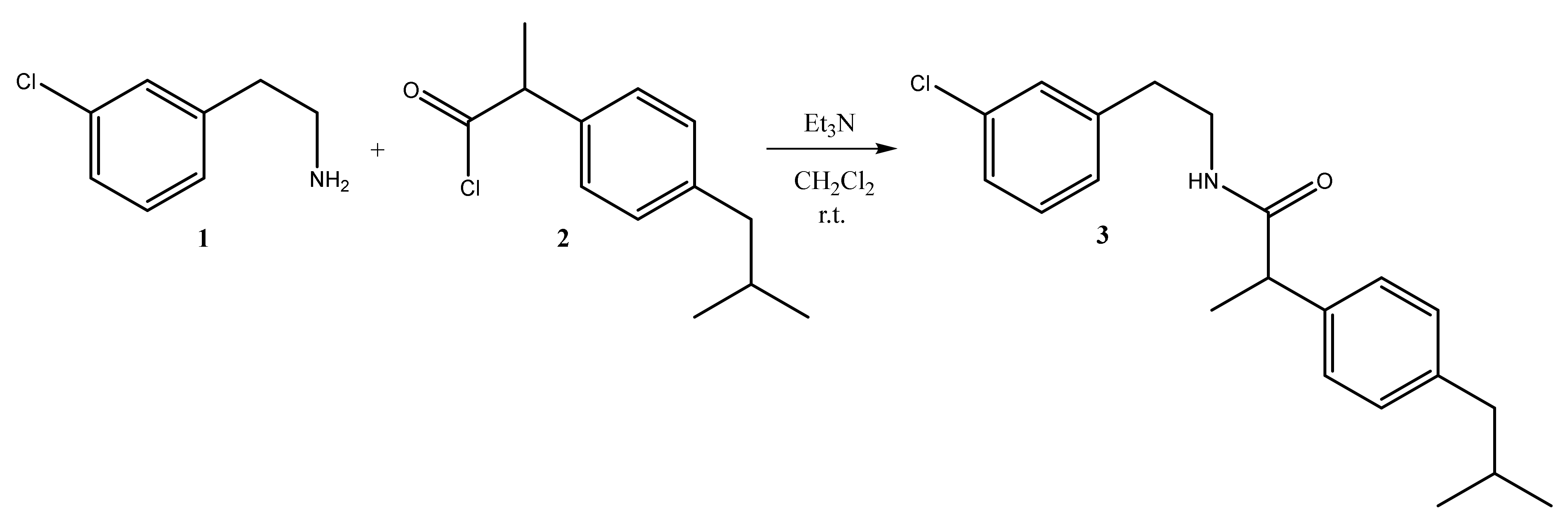
2. Results
3. Materials and Methods
3.1. Synthesis of 2-(4-Isobutylphenyl)propanoyl Chloride 2
3.2. Synthesis of N-(3-Chlorophenethyl)-2-(4-isobutylphenyl)propanamide 3
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naser, N.; Alibeg, A.; AbdAl-Zahra, A. Design, synthesis, in silico study and preliminary pharmacological evaluation of ibuprofen derivatives. Mater. Today Proc. 2022, 65, 2669–2675. [Google Scholar] [CrossRef]
- Algohary, A.; Hassan, M. Novel phospha-oxazepinoquinazolinyl derivatives of ibuprofen as nitric oxide synthase inhibitors: Synthesis and biological evaluation. Arab. J. Chem. 2022, 15, 103642. [Google Scholar] [CrossRef]
- Abbas, A.M.; Aboelmagd, A.; Kishk, S.M.; Nasrallah, H.H.; Boyd, W.C.; Kalil, H.; Orabi, A.S. A Novel Ibuprofen Derivative and Its Complexes: Physicochemical Characterization, DFT Modeling, Docking, In Vitro Anti-Inflammatory Studies, and DNA Interaction. Molecules 2022, 27, 7540. [Google Scholar] [CrossRef]
- Ashraf, Z.; Mahmood, T.; Hassan, M.; Afzal, S.; Rafique, H.; Afzal, K.; Latip, J. Dexibuprofen amide derivatives as potential anticancer agents: Synthesis, in silico docking, bioevaluation, and molecular dynamic simulation. Drug Des. Dev. Ther. 2019, 13, 1643–1657. [Google Scholar] [CrossRef]
- Chiodi, D.; Ishihara, Y. “Magic Chloro”: Profound Effects of the Chlorine Atom in Drug Discovery. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Shaik, A.B.; Bhandare, R.R.; Nissankararao, S.; Edis, Z.; Tangirala, N.R.; Shahanaaz, S.; Rahman, M.M. Design, Facile Synthesis and Characterization of Dichloro Substituted Chalcones and Dihydropyrazole Derivatives for Their Antifungal, Antitubercular and Antiproliferative Activities. Molecules 2020, 25, 3188. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolov, S.; Ivanov, I.; Bojilov, D.; Kalinova, Y. N-(3-Chlorophenethyl)-2-(4-isobutylphenyl)propanamide. Molbank 2023, 2023, M1536. https://doi.org/10.3390/M1536
Manolov S, Ivanov I, Bojilov D, Kalinova Y. N-(3-Chlorophenethyl)-2-(4-isobutylphenyl)propanamide. Molbank. 2023; 2023(1):M1536. https://doi.org/10.3390/M1536
Chicago/Turabian StyleManolov, Stanimir, Iliyan Ivanov, Dimitar Bojilov, and Yolina Kalinova. 2023. "N-(3-Chlorophenethyl)-2-(4-isobutylphenyl)propanamide" Molbank 2023, no. 1: M1536. https://doi.org/10.3390/M1536
APA StyleManolov, S., Ivanov, I., Bojilov, D., & Kalinova, Y. (2023). N-(3-Chlorophenethyl)-2-(4-isobutylphenyl)propanamide. Molbank, 2023(1), M1536. https://doi.org/10.3390/M1536








