Abstract
An efficient telescoped method for synthesis of 3-phenyl-10-(2,3,4-trimethoxyphenyl)-9,10-dihydro-4H,8H-pyrano[2,3-f]chromene-4,8-dione was elaborated. The presented protocol includes the one-pot multicomponent reaction of 7-hydroxy-3-phenyl-4H-chromen-4-one, 2,3,4-trimethoxybenzaldehyde and Meldrum’s acid. Advantages of this method are the application of readily available starting reagents, atom economy and easy procedure of preparation and purification of the target product. The structure of the synthesized polycyclic compound was proved by 1H, 13C-NMR, IR spectroscopy and high-resolution mass spectrometry with electrospray ionization (ESI-HRMS).
1. Introduction
Isoflavones are a broad class of natural products generally isolated from plant sources. These types of organic compounds are found almost exclusively in members of the bean family (Fabaceae). The application of isoflavones in medicine is of great interest due to their wide range of biological activity [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. It should be noted that the main use of this natural product is associated with their significant estrogenic properties. For example, preliminary research has shown that the employment of isoflavones results in a reduced risk of postmenopausal cancer [15,16,17,18] and osteoporosis in women [19]. In addition, soy isoflavones act as thyroid peroxidase blockers, leading to competitive inhibition of biosynthesis of the thyroid hormone [20,21,22]. The most well-known representatives of the considered class are shown in Figure 1.
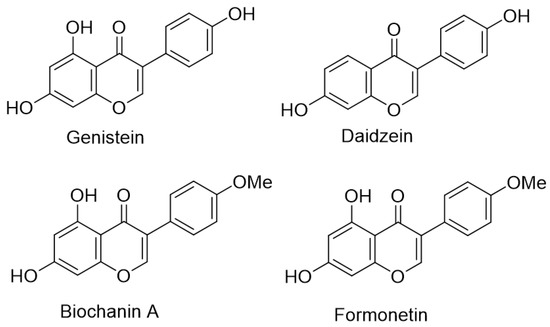
Figure 1.
Examples of bioactive isoflavones.
Taking into account the significant interest in the presented compounds for medicine, it can be assumed that complex products containing an isoflavone core will also demonstrate a variety of biological activities. In this regard, easily available isoflavones can be used as starting materials for the preparation of aforementioned systems. In addition, application of the multicomponent reaction methodology (MCRs) is a convenient general pathway for modification of various objects [23,24]. This approach makes it possible to obtain a wide range of products in one step, avoiding a complex sequence of multistage syntheses. Thus, the use of the MCRs method for derivatization of isoflavones opens access to a huge array of compounds with potential biological activity.
Previously, we elaborated a general route for the synthesis of condensed dihydropyranones based on a multicomponent reaction of 7-hydroxycoumarin derivatives with aldehydes and Meldrum’s acid [25]. The presented method is a two-stage telescoped protocol, including preliminary condensation of the starting components in methanol and subsequent heterocyclization in acetic acid. It should be noted that the presented approach makes it possible to prepare the wide range of coumarin-containing polycyclic products. It can be supposed that the considered method can be extended to the synthesis of the isoflavone derivatives.
2. Results
In the present paper, we disclose multicomponent condensation of 7-hydroxy-3-phenyl-4H-chromen-4-one (1), 2,3,4-trimethoxybenzaldehyde (2) and Meldrum’s acid (3), leading to previously undescribed 3-phenyl-10-(2,3,4-trimethoxyphenyl)-9,10-dihydro-4H,8H-pyrano [2,3-f]chromene-4,8-dione (4). We have demonstrated that the investigated synthesis is a telescoped two-step process performed in a one-pot format. The first stage includes the interaction of the starting materials in methanol using triethylamine as a basic catalyst, and this step was carried out at reflux for 3 h. It should be mentioned that for the complete conversion of the starting isoflavone 1 into the target product 4, 1.5-fold excess of the remaining reagents should be used. The final step of the process involves formation of target product 4, proceeding under reflux in acetic acid. As it was shown in a previous article, this two-step protocol is most efficient for the conversion of starting hydroxyl derivatives to corresponding dihydropyranones [25]. Thus, the described method allows one to obtain the target product 4 at a 68% yield (Scheme 1).
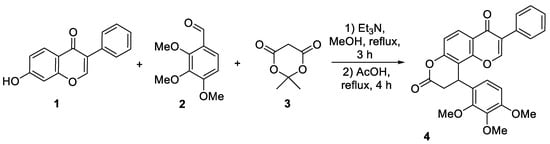
Scheme 1.
Synthesis of 3-phenyl-10-(2,3,4-trimethoxyphenyl)-9,10-dihydro-4H,8H-pyrano [2,3-f]chromene-4,8-dione 4.
It should be noted that the considered multicomponent condensation proceeds regiospecifically. A priori, we can suppose the formation of two isomeric products 4 and 5 as a result of the studied process (Figure 2). However, as a result of the condensation, only dihydropyranone 4 was obtained, and the formation of the isomer 5 in the reaction mixture was not detected.
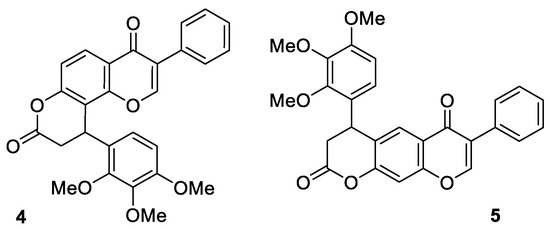
Figure 2.
Two isomeric products 4 and 5.
The obtained 3-phenyl-10-(2,3,4-trimethoxyphenyl)-9,10-dihydro-4H,8H-pyrano [2,3-f]chromene-4,8-dione (4) is a colorless crystalline compound, whose structure was proved by 1H, 13C NMR, IR spectroscopy and high-resolution mass spectrometry (See Supplementary Materials). The 1H NMR spectrum of the product 4 contains the characteristic doublet signal of the proton in the region δ 8.17 ppm, with a spin–spin coupling constant of 8.8 Hz, corresponding to the hydrogen atom in position five of the chromone system. Note that such a signal shape is impossible for the isomeric product 5, and thus based on 1H NMR data the supposed alternative structure of 5 was rejected.
The plausible reaction mechanism is presented in Scheme 2. At first, condensation of aldehyde 2 with Meldrum’s acid 3 results in the formation of a Michael acceptor A. Further, adduct C is produced by interaction of chromone anion B with intermediate A. Next, tautomerization of adduct C and cleavage of the 1,3-dioxane fragment under action of methanol leads to methyl ester E. Final acid-catalyzed intramolecular cyclization accompanied by release of methanol molecule results in target compound 4.
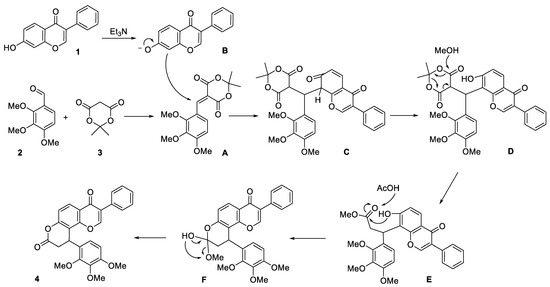
Scheme 2.
Proposed reaction mechanism for the formation of target product 4.
Note that the formation of alternative product 5 should proceed through the isomeric intermediate G (Scheme 3). At this stage it is probable that the addition at position six is energetically less favorable, due to the loss of aromaticity of the pyranone fragment in adduct G. Thus, the observed regiospecificity is a distinctive feature of the considered isoflavone 5.
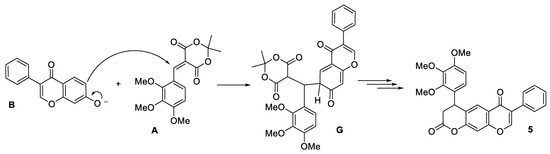
Scheme 3.
Proposed reaction mechanism for the formation of alternative product 5.
3. Materials and Methods
All starting chemicals and solvents were commercially available and were used as received. NMR spectra were recorded with Bruker Avance 300 (300 MHz) and Bruker DRX 500 (500 MHz) spectrometers (Billerica, MA, USA) in DMSO-d6. Chemical shifts (ppm) were given relative to solvent signals DMSO-d6: 2.50 ppm (1H NMR) and 39.52 ppm (13C NMR). High-resolution mass spectrum (HRMS) was obtained on a Bruker microTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (ESI). The melting point was determined on a Kofler hot stage (Dresden, Germany). IR spectrum was recorded on a Bruker ALPHA (Santa Barbara, CA, USA) spectrophotometer in a KBr pellet.
4. Conclusions
In summary, convenient one-pot telescoped protocol for the preparation of 3-phenyl-10-(2,3,4-trimethoxyphenyl)-9,10-dihydro-4H,8H-pyrano [2,3-f]chromene-4,8-dione was developed. The suggested method is based on multicomponent reaction of 7-hydroxy-3-phenyl-4H-chromen-4-one, 2,3,4-trimethoxybenzaldehyde and Meldrum’s acid. Advantages of the considered approach include the employment of readily available starting compounds, atom economy and easy work-up procedure. The structure of the obtained condensed dihydropyranone was confirmed by 1H, 13C-NMR, IR spectroscopy and high-resolution mass spectrometry with electrospray ionization (ESI-HRMS).
Experimental Procedure for the Synthesis of 3-phenyl-10-(2,3,4-trimethoxyphenyl)-9,10-dihydro-4H,8H-pyrano [2,3-f]chromene-4,8-dione 4
A mixture of 7-hydroxy-3-phenyl-4H-chromen-4-one 1 (0.48 g, 2 mmol), 2,3,4-trimethoxybenzaldehyde 2 (0.59 g, 3 mmol), Meldrum’s acid 3 (0.36 g, 3 mmol), and triethylamine (0.30 g, 3 mmol) in MeOH (10 mL) was refluxed for 3 h and the volatiles were removed in vacuo. To obtain residue, acetic acid (7 mL) was added and mixture was refluxed for 4 h. The obtained solution was evaporated in vacuo and residue was triturated at (4 mL). The precipitate formed was collected by filtration and washed with 2-propanole (3 × 5 mL). White powder; yield 68% (0.31 g); mp 163-165°. 1H NMR (300 MHz, DMSO-d6) (Figure S1) δ 8.52 (s, 1H), 8.17 (d, J = 8.8 Hz, 1H), 7.57 (d, J = 7.2 Hz, 2H), 7.48–7.33 (m, 4H), 6.69 (d, J = 8.7 Hz, 1H), 6.53 (d, J = 8.6 Hz, 1H), 5.04 (d, J = 7.7 Hz, 1H), 3.86 (s, 3H), 3.79 (s, 3H), 3.75 (s, 3H), 3.49 (dd, J = 16.3, 7.9 Hz, 1H), 2.81 (d, J = 16.2 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) (Figure S2) δ 174.43, 166.00, 155.37, 154.39, 153.19, 153.16, 150.63, 141.82, 131.44, 128.92, 128.15, 127.99, 126.26, 125.66, 124.07, 121.79, 120.59, 115.08, 113.57, 107.64, 60.70, 60.32, 55.77, 35.23, 29.99. IR spectrum (KBr), ν, cm−1 (Figure S3): 3701, 3654, 3603, 3582, 3544, 3502, 3470, 3432, 3413, 3393, 3367, 3337, 3310, 3270, 3233, 3155, 3119, 2978, 2948, 2845, 2390, 2349, 1956, 1909, 1791, 1641, 1601, 1494, 1466, 1439, 1418, 1375, 1335, 1291, 1274, 1262, 1236, 11207, 1187, 1126, 1093, 1032, 1000, 974, 907, 869, 799. HRMS (ESI-TOF) (Figure S4) m/z: [M+H]+ Calcld for C27H22O7+H+: 459.1438; Found: 459.1429.
Supplementary Materials
The following are available online: copies of 1H, 13C-NMR, mass and IR spectra for compound 4. Figure S1: 1H NMR spectrum (300 MHz) of compound 4 in DMSO-d6; Figure S2: 13C {1H} NMR spectrum (126 MHz) of compound 4 in DMSO-d6; Figure S3: HRMS for compound 4; Figure S4: IR spectrum for compound 4.
Author Contributions
B.V.L.—conceptualization, synthesis, spectroscopic analysis and writing the manuscript. A.N.K.—conceptualization, synthesis, spectroscopic analysis and writing the manuscript. V.G.M.—conceptualization, synthesis, spectroscopic analysis and writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for the compounds presented in this study are available in the Supplementary Materials of this article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Sample of the compound 4 is available from the authors.
References
- Blicharski, T.; Oniszczuk, A. Extraction Methods for the Isolation of Isoflavonoids from Plant Material. Open Chem. 2017, 15, 34–45. [Google Scholar] [CrossRef]
- Bustamante-Rangel, M.; Delgado-Zamarreño, M.M.; Pérez-Martín, L.; Rodríguez-Gonzalo, E.; Domínguez-Álvarez, J. Analysis of Isoflavones in Foods: Analysis of Isoflavones in Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G. Isoflavone Research towards Healthcare Applications. J. Cancer Metastasis Treat. 2020, 6, 48. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Dulce-María, D.-A.; Adrián, C.-R.; Cuauhtémoc, R.-M.; Ada-Keila, M.-N.; Jorge, M.-C.; Erika, A.-S.; Edith-Oliva, C.-R. Isoflavones from Black Chickpea (Cicer arietinum L.) Sprouts with Antioxidant and Antiproliferative Activity. Saudi J. Biol. Sci. 2021, 28, 1141–1146. [Google Scholar] [CrossRef]
- Ko, K.-P. Isoflavones: Chemistry, Analysis, Functions and Effects on Health and Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 7001–7010. [Google Scholar] [CrossRef]
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic Engineering of Isoflavones: An Updated Overview. Front. Plant Sci. 2021, 12, 670103. [Google Scholar] [CrossRef]
- Munro, I.C.; Harwood, M.; Hlywka, J.J.; Stephen, A.M.; Doull, J.; Flamm, W.G.; Adlercreutz, H. Soy Isoflavones: A Safety Review. Nutr. Rev. 2003, 61, 1–33. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, C.; Momma, H.; Niu, K.; Nagatomi, R. Daily Dietary Isoflavone Intake in Relation to Lowered Risk of Depressive Symptoms among Men. J. Affect. Disord. 2020, 261, 121–125. [Google Scholar] [CrossRef]
- Messina, M.; Mejia, S.B.; Cassidy, A.; Duncan, A.; Kurzer, M.; Nagato, C.; Ronis, M.; Rowland, I.; Sievenpiper, J.; Barnes, S. Neither Soyfoods nor Isoflavones Warrant Classification as Endocrine Disruptors: A Technical Review of the Observational and Clinical Data. Crit. Rev. Food Sci. Nutr. 2022, 62, 5824–5885. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Jo, A.; Casale, T.; Jeong, S.J.; Hong, S.-J.; Cho, J.K.; Holbrook, J.T.; Kumar, R.; Smith, L.J. Soy Isoflavones Reduce Asthma Exacerbation in Asthmatic Patients with High PAI-1–Producing Genotypes. J. Allergy Clin. Immunol. 2019, 144, 109–117.e4. [Google Scholar] [CrossRef]
- Wang, Q.; Ge, X.; Tian, X.; Zhang, Y.; Zhang, J.; Zhang, P. Soy Isoflavone: The Multipurpose Phytochemical (Review). Biomed. Rep. 2013, 1, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, Y.; Li, Y.; Li, Y.; Feng, C.; Li, Z. Daidzein-Rich Isoflavones Aglycone Inhibits Lung Cancer Growth through Inhibition of NF-ΚB Signaling Pathway. Immunol. Lett. 2020, 222, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Varinska, L.; Gal, P.; Mojzisova, G.; Mirossay, L.; Mojzis, J. Soy and Breast Cancer: Focus on Angiogenesis. Int. J. Mol. Sci. 2015, 16, 11728–11749. [Google Scholar] [CrossRef]
- Jacobs, A.; Wegewitz, U.; Sommerfeld, C.; Grossklaus, R.; Lampen, A. Efficacy of Isoflavones in Relieving Vasomotor Menopausal Symptoms—A Systematic Review. Mol. Nutr. Food Res. 2009, 53, 1084–1097. [Google Scholar] [CrossRef]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef]
- Qiu, S.; Jiang, C. Soy and Isoflavones Consumption and Breast Cancer Survival and Recurrence: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2019, 58, 3079–3090. [Google Scholar] [CrossRef]
- Wei, P.; Liu, M.; Chen, Y.; Chen, D.-C. Systematic Review of Soy Isoflavone Supplements on Osteoporosis in Women. Asian Pac. J. Trop. Med. 2012, 5, 243–248. [Google Scholar] [CrossRef]
- Divi, R.L.; Chang, H.C.; Doerge, D.R. Anti-Thyroid Isoflavones from Soybean. Biochem. Pharmacol. 1997, 54, 1087–1096. [Google Scholar] [CrossRef]
- Divi, R.L.; Doerge, D.R. Inhibition of Thyroid Peroxidase by Dietary Flavonoids. Chem. Res. Toxicol. 1996, 9, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Giuliani, C. Plant Constituents and Thyroid: A Revision of the Main Phytochemicals That Interfere with Thyroid Function. Food Chem. Toxicol. 2021, 152, 112158. [Google Scholar] [CrossRef] [PubMed]
- Younus, H.A.; Al-Rashida, M.; Hameed, A.; Uroos, M.; Salar, U.; Rana, S.; Khan, K.M. Multicomponent Reactions (MCR) in Medicinal Chemistry: A Patent Review (2010-2020). Expert Opin. Ther. Pat. 2021, 31, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Graebin, C.S.; Ribeiro, F.V.; Rogério, K.R.; Kümmerle, A.E. Multicomponent Reactions for the Synthesis of Bioactive Compounds: A Review. Curr. Org. Synth. 2019, 16, 855–899. [Google Scholar] [CrossRef] [PubMed]
- Lichitsky, B.V.; Shorunov, S.V.; Osipov, A.O.; Komogortsev, A.N.; Dudinov, A.A.; Krayushkin, M.M. Multicomponent Condensation of 6-Acetyl-5,7-Dihydroxy-4-Methylchromen-2-One with Aldehydes and Meldrum’s Acid. Russ. Chem. Bull. 2017, 66, 886–890. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).