5-(4-Nitrophenyl)furan-2-carboxylic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
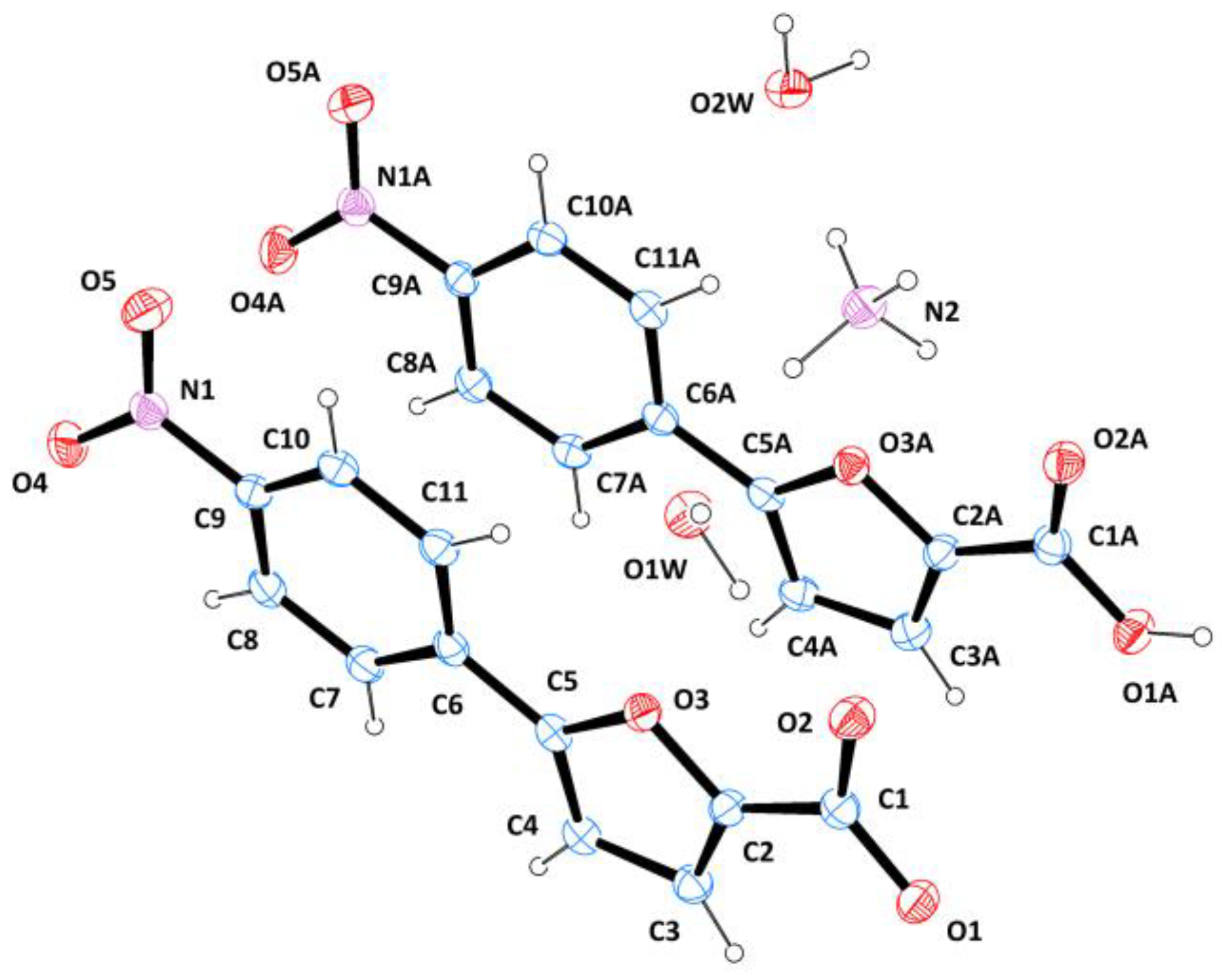
2.2. Crystallization and Structure Determination
- di: distance between the HS and the nearest nucleus inside the surface
- de: distance between the HS and the nearest nucleus outside the surface
- rvdW: van der Waals radius of the atom.
3. Materials and Methods
3.1. Chemistry
3.2. X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789240037021.
- Cazzaniga, G.; Mori, M.; Chiarelli, L.R.; Gelain, A.; Meneghetti, F.; Villa, S. Natural products against key Mycobacterium tuberculosis enzymatic targets: Emerging opportunities for drug discovery. Eur. J. Med. Chem. 2021, 224, 113732. [Google Scholar] [CrossRef] [PubMed]
- Ejalonibu, M.A.; Ogundare, S.A.; Elrashedy, A.A.; Ejalonibu, M.A.; Lawal, M.M.; Mhlongo, N.N.; Kumalo, H.M. Drug Discovery for Mycobacterium tuberculosis Using Structure-Based Computer-Aided Drug Design Approach. Int. J. Mol. Sci. 2021, 22, 13259. [Google Scholar] [CrossRef] [PubMed]
- Motamen, S.; Quinn, R.J. Analysis of Approaches to Anti-tuberculosis Compounds. ACS Omega 2020, 5, 28529–28540. [Google Scholar] [CrossRef] [PubMed]
- Buroni, S.; Chiarelli, L.R. Antivirulence compounds: A future direction to overcome antibiotic resistance? Future Microbiol. 2020, 15, 299–301. [Google Scholar] [CrossRef]
- Chiarelli, L.R.; Mori, M.; Barlocco, D.; Beretta, G.; Gelain, A.; Pini, E.; Porcino, M.; Mori, G.; Stelitano, G.; Costantino, L.; et al. Discovery and Development of Novel Salicylate Synthase (MbtI) Furanic Inhibitors as Antitubercular Agents. Eur. J. Med. Chem. 2018, 155, 754–763. [Google Scholar] [CrossRef]
- Chao, A.; Sieminski, P.J.; Owens, C.P.; Goulding, C.W. Iron Acquisition in Mycobacterium tuberculosis. Chem. Rev. 2019, 119, 1193–1220. [Google Scholar] [CrossRef]
- Shyam, M.; Shilkar, D.; Rakshit, G.; Jayaprakash, V. Approaches for targeting the mycobactin biosynthesis pathway for novel anti-tubercular drug discovery: Where we stand. Expert Opin. Drug Discov. 2022, 17, 699–715. [Google Scholar] [CrossRef]
- Khurana, H.; Srivastava, M.; Chaudhary, D.; Gosain, T.P.; Kumari, R.; Bean, A.C.; Chugh, S.; Maiti, T.K.; Stephens, C.E.; Asthana, S.; et al. Identification of diphenyl furan derivatives via high throughput and computational studies as ArgA inhibitors of Mycobacterium tuberculosis. Int. J. Biol. Macromol. 2021, 193, 1845–1858. [Google Scholar] [CrossRef]
- Metaferia, B.B.; Fetterolf, B.J.; Shazad-ul-Hussan, S.; Moravec, M.; Smith, J.A.; Ray, S.; Gutierrez-Lugo, M.T.; Bewley, C.A. Synthesis of natural product-inspired inhibitors of Mycobacterium tuberculosis mycothiol-associated enzymes: The first inhibitors of GlcNAc-Ins deacetylase. J. Med. Chem. 2007, 50, 6326–6336. [Google Scholar] [CrossRef]
- Reshma, R.S.; Jeankumar, V.U.; Kapoor, N.; Saxena, S.; Bobesh, K.A.; Vachaspathy, A.R.; Kolattukudy, P.E.; Sriram, D. Mycobacterium tuberculosis lysine-ɛ-aminotransferase a potential target in dormancy: Benzothiazole based inhibitors. Bioorg. Med. Chem. 2017, 25, 2761–2771. [Google Scholar] [CrossRef]
- Manger, M.; Scheck, M.; Prinz, H.; Von Kries, J.P.; Langer, T.; Saxena, K.; Schwalbe, H.; Fürstner, A.; Rademann, J.; Waldmann, H. Discovery of Mycobacterium Tuberculosis Protein Tyrosine Phosphatase A (MptpA) Inhibitors Based on Natural Products and a Fragment-Based Approach. ChemBioChem 2005, 6, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Sens, L.; de Souza, A.C.A.; Pacheco, L.A.; Menegatti, A.C.O.; Mori, M.; Mascarello, A.; Nunes, R.J.; Terenzi, H. Synthetic thiosemicarbazones as a new class of Mycobacterium tuberculosis protein tyrosine phosphatase A inhibitors. Bioorg. Med. Chem. 2018, 26, 5742–5750. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, N.L.; Lipker, L.; Hanson, A.M.; Bohl, C.J.; Engel, K.E.; Kalous, K.S.; Stemper, M.E.; Sem, D.S.; Schwan, W.R. Docking into Mycobacterium tuberculosis Thioredoxin Reductase Protein Yields Pyrazolone Lead Molecules for Methicillin-Resistant Staphylococcus aureus. Antibiotics 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Verbitskiy, E.V.; Baskakova, S.A.; Belyaev, D.V.; Vakhrusheva, D.V.; Eremeeva, N.I.; Rusinov, G.L.; Charushin, V.N. Renaissance of 4-(5-nitrofuran-2-yl)-5-arylamino substituted pyrimidines: Microwave-assisted synthesis and antitubercular activity. Mendeleev Commun. 2021, 31, 210–212. [Google Scholar] [CrossRef]
- Elsaman, T.; Mohamed, M.S.; Mohamed, M.A. Current development of 5-nitrofuran-2-yl derivatives as antitubercular agents. Bioorg. Chem. 2019, 88, 102969. [Google Scholar] [CrossRef]
- Faazil, S.; Malik, M.S.; Ahmed, S.A.; Alsantali, R.I.; Yedla, P.; Alsharif, M.A.; Shaikh, I.N.; Kamal, A. Novel linezolid-based oxazolidinones as potent anticandidiasis and antitubercular agents. Bioorg. Chem. 2022, 126, 105869. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Baskakova, S.A.; Gerasimova, N.A.; Evstigneeva, N.P.; Zil’berberg, N.V.; Kungurov, N.V.; Kravchenko, M.A.; Rusinov, G.L.; Chupakhina, O.N.; Charushin, V.N. New 5-arylamino-4-(5-nitrofuran-2-yl)pyrimidines as promising antibacterial agents. Mendeleev Commun. 2018, 28, 393–395. [Google Scholar] [CrossRef]
- Kaur, S.; Nieto, N.S.; McDonald, P.; Beck, J.R.; Honzatko, R.B.; Roy, A.; Nelson, S.W. Discovery of small molecule inhibitors of Plasmodium falciparum apicoplast DNA polymerase. J. Enzyme Inhib. Med. Chem. 2022, 37, 1320–1326. [Google Scholar] [CrossRef]
- Chiarelli, L.R.; Mori, M.; Beretta, G.; Gelain, A.; Pini, E.; Sammartino, J.C.; Stelitano, G.; Barlocco, D.; Costantino, L.; Lapillo, M.; et al. New Insight into Structure-Activity of Furan-based Salicylate Synthase (MbtI) Inhibitors as Potential Antitubercular Agents. J. Enzyme Inhib. Med. Chem. 2019, 34, 823–828. [Google Scholar] [CrossRef]
- Mori, M.; Stelitano, G.; Gelain, A.; Pini, E.; Chiarelli, L.R.; Sammartino, J.C.; Poli, G.; Tuccinardi, T.; Beretta, G.; Porta, A.; et al. Shedding X-ray Light on the Role of Magnesium in the Activity of M. tuberculosis Salicylate Synthase (MbtI) for Drug Design. J. Med. Chem. 2020, 63, 7066–7080. [Google Scholar] [CrossRef]
- Mori, M.; Stelitano, G.; Chiarelli, L.R.; Cazzaniga, G.; Gelain, A.; Barlocco, D.; Pini, E.; Meneghetti, F.; Villa, S. Synthesis, Characterization, and Biological Evaluation of New Derivatives Targeting MbtI as Antitubercular Agents. Pharmaceuticals 2021, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Stelitano, G.; Griego, A.; Chiarelli, L.R.; Cazzaniga, G.; Gelain, A.; Pini, E.; Camera, M.; Canzano, P.; Fumagalli, A.; et al. Synthesis and Assessment of the In Vitro and Ex Vivo Activity of Salicylate Synthase (Mbti) Inhibitors as New Candidates for the Treatment of Mycobacterial Infections. Pharmaceuticals 2022, 15, 992. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Motherwell, W.D.S.; Purkis, L.H.; Smith, B.R.; Taylor, R.; Cooper, R.I.; Harris, S.E.; et al. Retrieval of Crystallographically-Derived Molecular Geometry Information. J. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, M. PARST 95—An update to PARST: A system of Fortran routines for calculating molecular structure parameters from the results of crystal structure analyses. J. Appl. Crystallogr. 1995, 28, 659. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Mori, M.; Meneghetti, F.; Chiarelli, L.R.; Stelitano, G.; Caligiuri, I.; Rizzolio, F.; Ciceri, S.; Poli, G.; Staver, D.; et al. Virtual screening and crystallographic studies reveal an unexpected γ-lactone derivative active against MptpB as a potential antitubercular agent. Eur. J. Med. Chem. 2022, 234, 114235. [Google Scholar] [CrossRef]
- Jelsch, C.; Ejsmont, K.; Huder, L. The enrichment ratio of atomic contacts in crystals, an indicator derived from the Hirshfeld surface analysis. IUCrJ 2014, 1, 119–128. [Google Scholar] [CrossRef]
- Weber, P.; Pissis, C.; Navaza, R.; Mechaly, A.E.; Saul, F.; Alzari, P.M.; Haouz, A. High-Throughput Crystallization Pipeline at the Crystallography Core Facility of the Institut Pasteur. Molecules 2019, 24, 4451. [Google Scholar] [CrossRef]
- Nanao, M.; Basu, S.; Zander, U.; Giraud, T.; Surr, J.; Guijarro, M.; Lentini, M.; Felisaz, F.; Sinoir, J.; Morawe, C.; et al. ID23-2: An automated and high-performance microfocus beamline for macromolecular crystallography at the ESRF. J. Synchrotron Radiat. 2022, 29, 581–590. [Google Scholar] [CrossRef]
- Kabsch, W. IUCr XDS. Acta Crystallogr. Sect. D Struct. Biol. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]

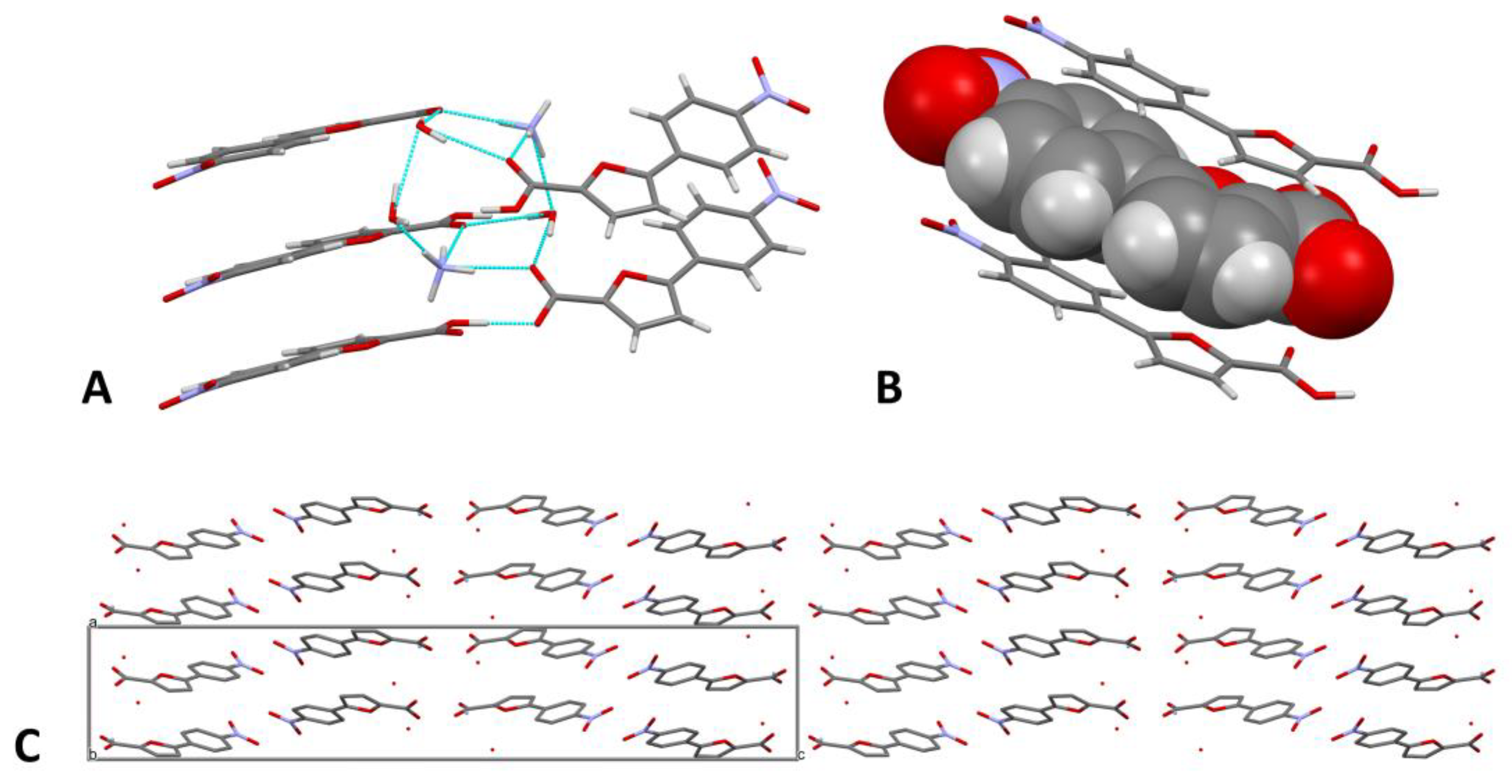
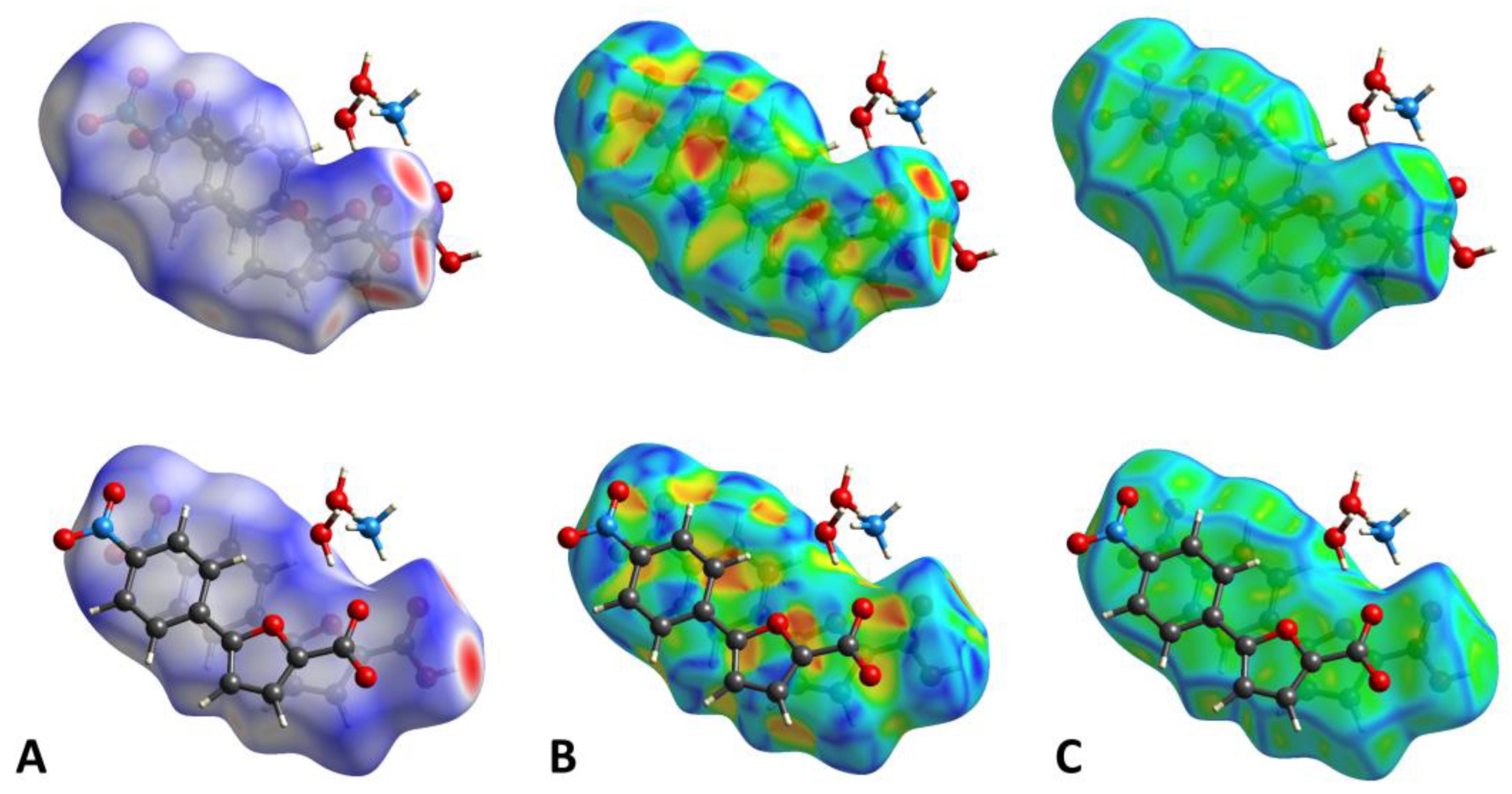
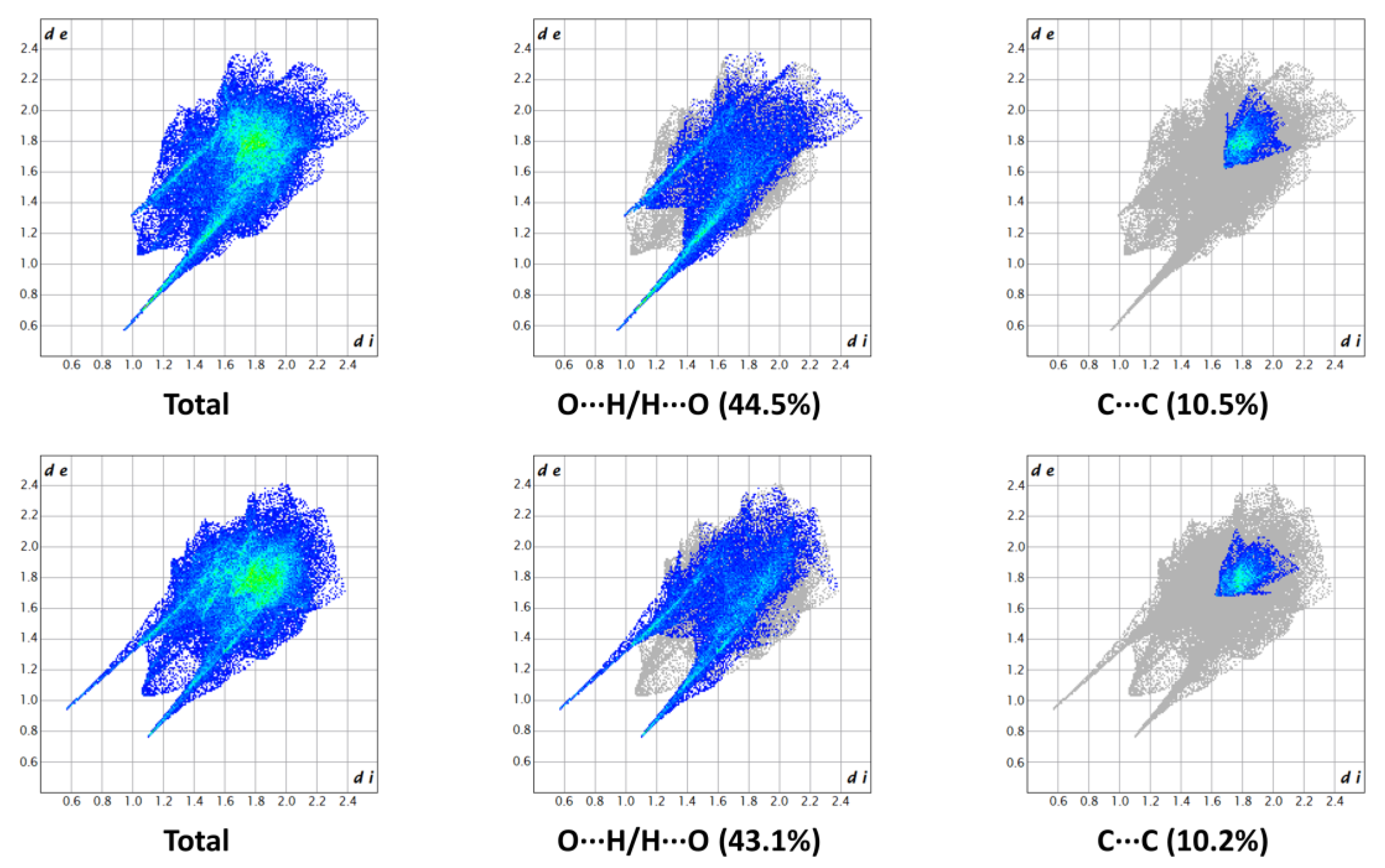
| H-Bond | D-H/Å | H∙∙∙A/Å | D∙∙∙A/Å | D-H∙∙∙A/° |
|---|---|---|---|---|
| O1W-H1W···O2 0 | 0.976(4) | 1.788(4) | 2.753(2) | 169(4) |
| C11-H11···O1W 0 | 0.930(2) | 2.731(2) | 3.657(3) | 174(1) |
| N2-H2A···O2W 0 | 0.88(3) | 1.89(3) | 2.773(3) | 176(3) |
| N2-H2B···O1W 0 | 0.99(3) | 1.89(3) | 2.862(3) | 166(3) |
| N2-H2C···O2A 0 | 0.94(4) | 1.95(4) | 2.859(3) | 162(3) |
| C11A-H11A···O2W 0 | 0.930(2) | 2.527(2) | 3.304(3) | 141(1) |
| C3-H3···O2W I | 0.930(2) | 2.459(2) | 3.270(3) | 146(1) |
| C4-H4···O5A I | 0.930(2) | 2.536(2) | 3.357(3) | 147(1) |
| O1A-H1A···O1 II | 0.96(4) | 1.55(4) | 2.498(2) | 171(3) |
| N2-H2D···O2 III | 0.94(3) | 1.83(3) | 2.765(3) | 168(3) |
| O1W-H2W···O2A IV | 0.85(4) | 2.03(4) | 2.856(2) | 165(4) |
| O2W-H3W···O1 V | 0.87(4) | 1.88(4) | 2.742(2) | 167(4) |
| O2W-H4W···O1W VI | 0.98(4) | 1.89(4) | 2.833(3) | 161(4) |
| 1 | V (Å3) | A (Å2) | G | Ω |
|---|---|---|---|---|
| HS-0 | 233.21 | 241.21 | 0.760 | 0.314 |
| HS-A | 244.00 | 247.39 | 0.763 | 0.341 |
| Parameter | Data | |
|---|---|---|
| Identification code | 1 | |
| Empirical formula | C22H21N3O12 | |
| Formula weight | 519.42 | |
| Temperature | 100(2) K | |
| Crystal system | Orthorhombic | |
| Space group | P212121 | |
| Unit cell dimensions | a = 7.34 Å | α = 90° |
| b = 7.97 Å | β = 90° | |
| c = 39.21 Å | γ = 90° | |
| Volume | 2293.8 Å3 | |
| Z | 4 | |
| Density (calculated) | 1.504 Mg/m3 | |
| Absorption coefficient | 0.125 mm−1 | |
| F(000) | 1080 | |
| Crystal size | 0.1 × 0.02 × 0.01 mm3 | |
| θ range for data collection | 1.039 to 28.431° | |
| Index ranges | −8 ≤ h ≤ 8, −10 ≤ k ≤ 10, −47 ≤ l ≤ 47 | |
| Reflections collected | 29,316 | |
| Independent reflections | 5065 [R(int) = 0.0553] | |
| Refinement method | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 5065/0/370 | |
| Goodness-of-fit on F2 | 1.039 | |
| Final R indices [I > 2σ(I)] | R1 = 0.0348, wR2 = 0.0836 | |
| R indices (all data) | R1 = 0.0407, wR2 = 0.0878 | |
| Largest diff. peak and hole | 0.319 and −0.214 eÅ−3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, M.; Tresoldi, A.; Villa, S.; Cazzaniga, G.; Bellinzoni, M.; Meneghetti, F. 5-(4-Nitrophenyl)furan-2-carboxylic Acid. Molbank 2022, 2022, M1515. https://doi.org/10.3390/M1515
Mori M, Tresoldi A, Villa S, Cazzaniga G, Bellinzoni M, Meneghetti F. 5-(4-Nitrophenyl)furan-2-carboxylic Acid. Molbank. 2022; 2022(4):M1515. https://doi.org/10.3390/M1515
Chicago/Turabian StyleMori, Matteo, Andrea Tresoldi, Stefania Villa, Giulia Cazzaniga, Marco Bellinzoni, and Fiorella Meneghetti. 2022. "5-(4-Nitrophenyl)furan-2-carboxylic Acid" Molbank 2022, no. 4: M1515. https://doi.org/10.3390/M1515
APA StyleMori, M., Tresoldi, A., Villa, S., Cazzaniga, G., Bellinzoni, M., & Meneghetti, F. (2022). 5-(4-Nitrophenyl)furan-2-carboxylic Acid. Molbank, 2022(4), M1515. https://doi.org/10.3390/M1515







