Bis(3,4-diphenyl)(2-methythienyl)cyclopentadienyl Terbium Chloride
Abstract
1. Introduction
2. Results and Discussion
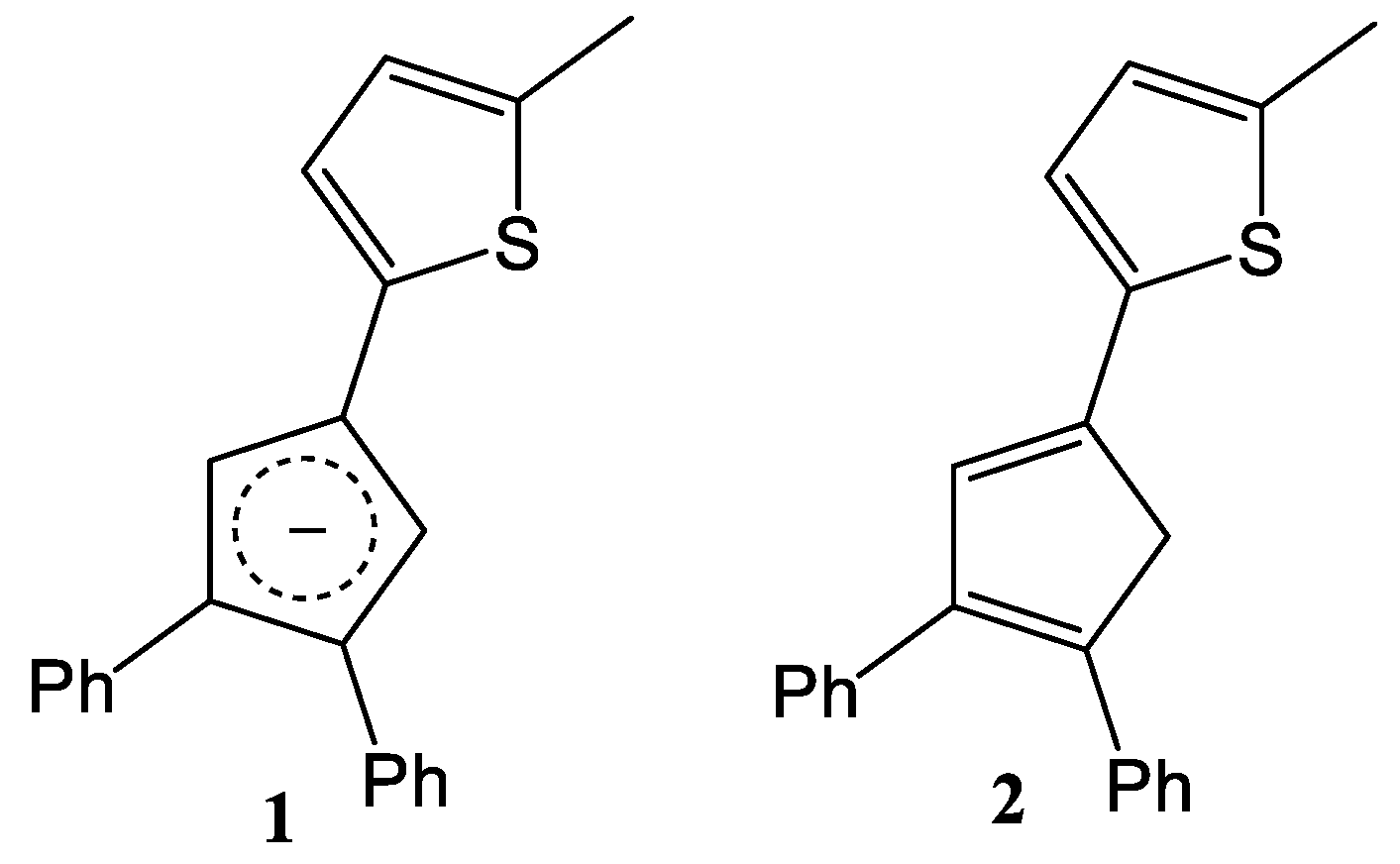
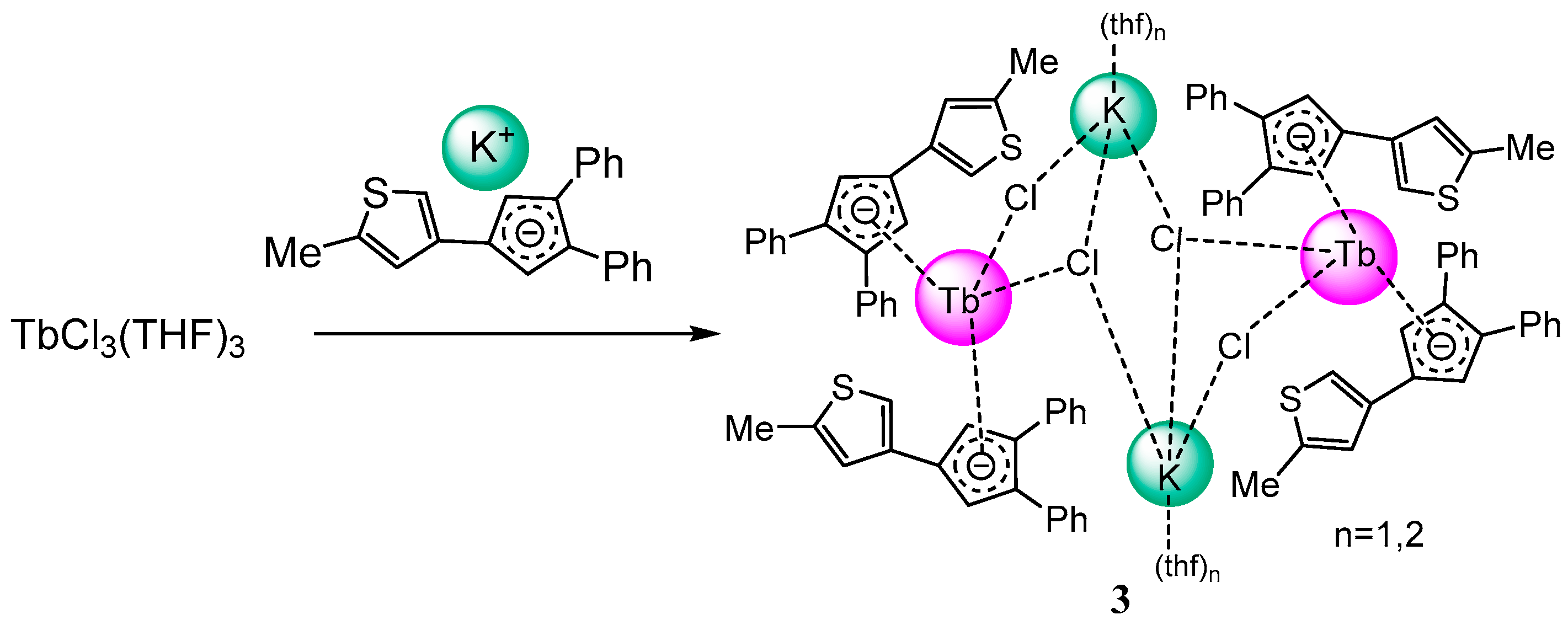
2.1. Synthesis and Structure
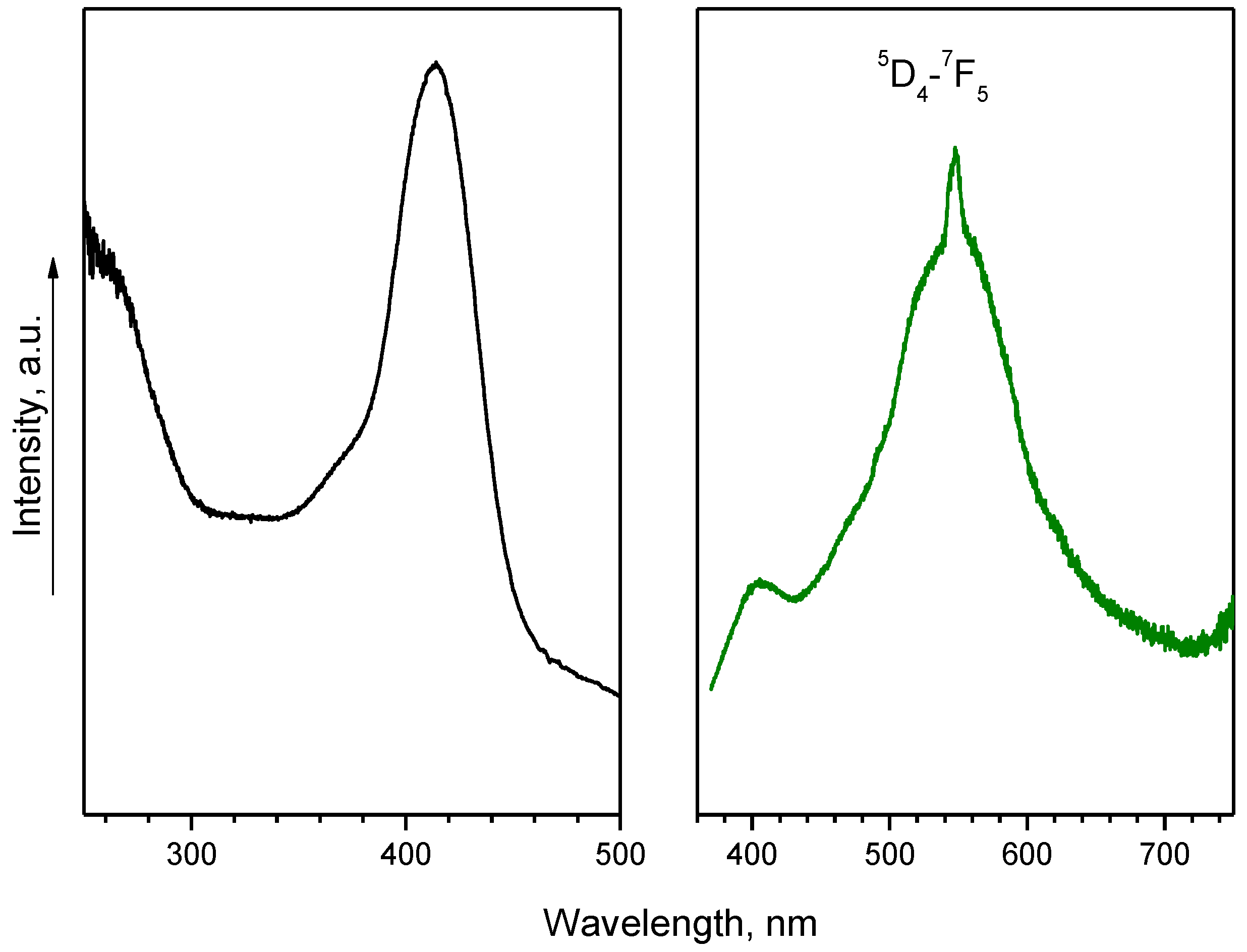
2.2. Photophysical Properties
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis
3.2.1. 2-(3,4-diphenylcyclopenta-1,3-dien-1-yl)-5-methylthiophene (2)
3.2.2. Bis-(3,4-diphenyl)(2-methylthienyl)cyclopentadienyl-terbium-dichloro-potassium-tetrahydrofuranate (3)
3.3. X-ray Diffraction Studies
3.4. Optical Measurments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, Z.; Wakatsuki, Y. Recent Developments in Organolanthanide Polymerization Catalysts. Coord. Chem. Rev. 2002, 231, 1–22. [Google Scholar] [CrossRef]
- Birmingham, J.M.; Wilkinson, G. The Cyclopentadienides of Scandium, Yttrium and Some Rare Earth Elements. J. Am. Chem. Soc. 1956, 78, 42–44. [Google Scholar] [CrossRef]
- Manastyrskyj, S.; Maginn, R.E.; Dubeck, M. The Preparation of Cyclopentadienyllanthanide Dichlorides. Inorg. Chem. 1963, 2, 904–905. [Google Scholar] [CrossRef]
- Maginn, R.E.; Manastyrskyj, S.; Dubeck, M. The Dicyclopentadienyllanthanide Chlorides. J. Am. Chem. Soc. 1963, 85, 672–676. [Google Scholar] [CrossRef]
- Wedal, J.C.; Evans, W.J. A Rare-Earth Metal Retrospective to Stimulate All Fields. J. Am. Chem. Soc. 2021, 143, 18354–18367. [Google Scholar] [CrossRef] [PubMed]
- Cooper, O.J.; Mills, D.P.; Lewis, W.; Blake, A.J.; Liddle, S.T. Reactivity of the Uranium(IV) Carbene Complex [U(BIPMTMS)(Cl)(μ-Cl)2Li(THF)2](BIPMTMS = {C(PPh2NSiMe3)2}) towards Carbonyl and Heteroallene Substrates. Dalt. Trans. 2014, 43, 14275–14283. [Google Scholar] [CrossRef] [PubMed]
- Harder, S.; Naglav, D.; Ruspic, C.; Wickleder, C.; Adlung, M.; Hermes, W.; Eul, M.; Pöttgen, R.; Rego, D.B.; Poineau, F.; et al. Physical Properties of Superbulky Lanthanide Metallocenes: Synthesis and Extraordinary Luminescence of [Eu II (CpBIG)2] (Cp BIG =(4- n Bu-C6H4)5-Cyclopentadienyl). Chem.-A Eur. J. 2013, 19, 12272–12280. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.P.; Bell, T.D.M.; Cox, R.P.; Daniels, D.P.; Deacon, G.B.; Jaroschik, F.; Junk, P.C.; Le Goff, X.F.; Lemercier, G.; Martinez, A.; et al. Divalent Tetra- and Penta-Phenylcyclopentadienyl Europium and Samarium Sandwich and Half-Sandwich Complexes: Synthesis, Characterization, and Remarkable Luminescence Properties. Organometallics 2015, 34, 5624–5636. [Google Scholar] [CrossRef]
- Evans, W.J. Tutorial on the Role of Cyclopentadienyl Ligands in the Discovery of Molecular Complexes of the Rare-Earth and Actinide Metals in New Oxidation States. Organometallics 2016, 35, 3088–3100. [Google Scholar] [CrossRef]
- Evans, W.J. The Organometallic Chemistry of the Lanthanide Elements in Low Oxidation States. Polyhedron 1987, 6, 803–835. [Google Scholar] [CrossRef]
- Roitershtein, D.M.; Puntus, L.N.; Vinogradov, A.A.; Lyssenko, K.A.; Minyaev, M.E.; Dobrokhodov, M.D.; Taidakov, I.V.; Varaksina, E.A.; Churakov, A.V.; Nifant’ev, I.E. Polyphenylcyclopentadienyl Ligands as an Effective Light-Harvesting μ-Bonded Antenna for Lanthanide +3 Ions. Inorg. Chem. 2018, 57, 10199–10213. [Google Scholar] [CrossRef] [PubMed]
- Minyaev, M.E.; Komarov, P.D.; Roitershtein, D.M.; Lyssenko, K.A.; Nifant’ev, I.E.; Puntus, L.N.; Varaksina, E.A.; Borisov, R.S.; Dyadchenko, V.P.; Ivchenko, P.V. Aryloxy Alkyl Magnesium versus Dialkyl Magnesium in the Lanthanidocene-Catalyzed Coordinative Chain Transfer Polymerization of Ethylene. Organometallics 2019, 38, 2892–2901. [Google Scholar] [CrossRef]
- Vinogradov, A.A.; Komarov, P.D.; Puntus, L.N.; Taydakov, I.V.; Lyssenko, K.A.; Nifant’ev, I.E.; Varaksina, E.A.; Roitershtein, D.M. Luminescence Sensitization of the Nd3+ Ion in Diphenyl(9-Antnracenyl)Cyclopentadienyl Complexes Containing Antenna-Ligand with Extended π-System. Inorg. Chim. Acta 2022, 533, 120777. [Google Scholar] [CrossRef]
- Varaksina, E.A.; Kiskin, M.A.; Lyssenko, K.A.; Puntus, L.N.; Korshunov, V.M.; Silva, G.S.; Freire, R.O.; Taydakov, I.V. Tuning the Luminescence Efficiency by Perfluorination of Side Chains in Eu3+complexes with β-Diketones of the Thiophene Series. Phys. Chem. Chem. Phys. 2021, 23, 25748–25760. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, F.T.; Poremba, P. Synthetic Methods of Organometallic and Inorganic Chemistry (Herrman/Brauer); Edelmann, F.T., Herrmann, W.A., Eds.; Verlag: Stuttgart, Germany, 1997; pp. 34–35. [Google Scholar]
- Lochmann, L.; Trekoval, J. Lithium-Potassium Exchange in Alkyllithium/Potassium t-Pentoxide Systems. J. Organomet. Chem. 1987, 326, 1–7. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
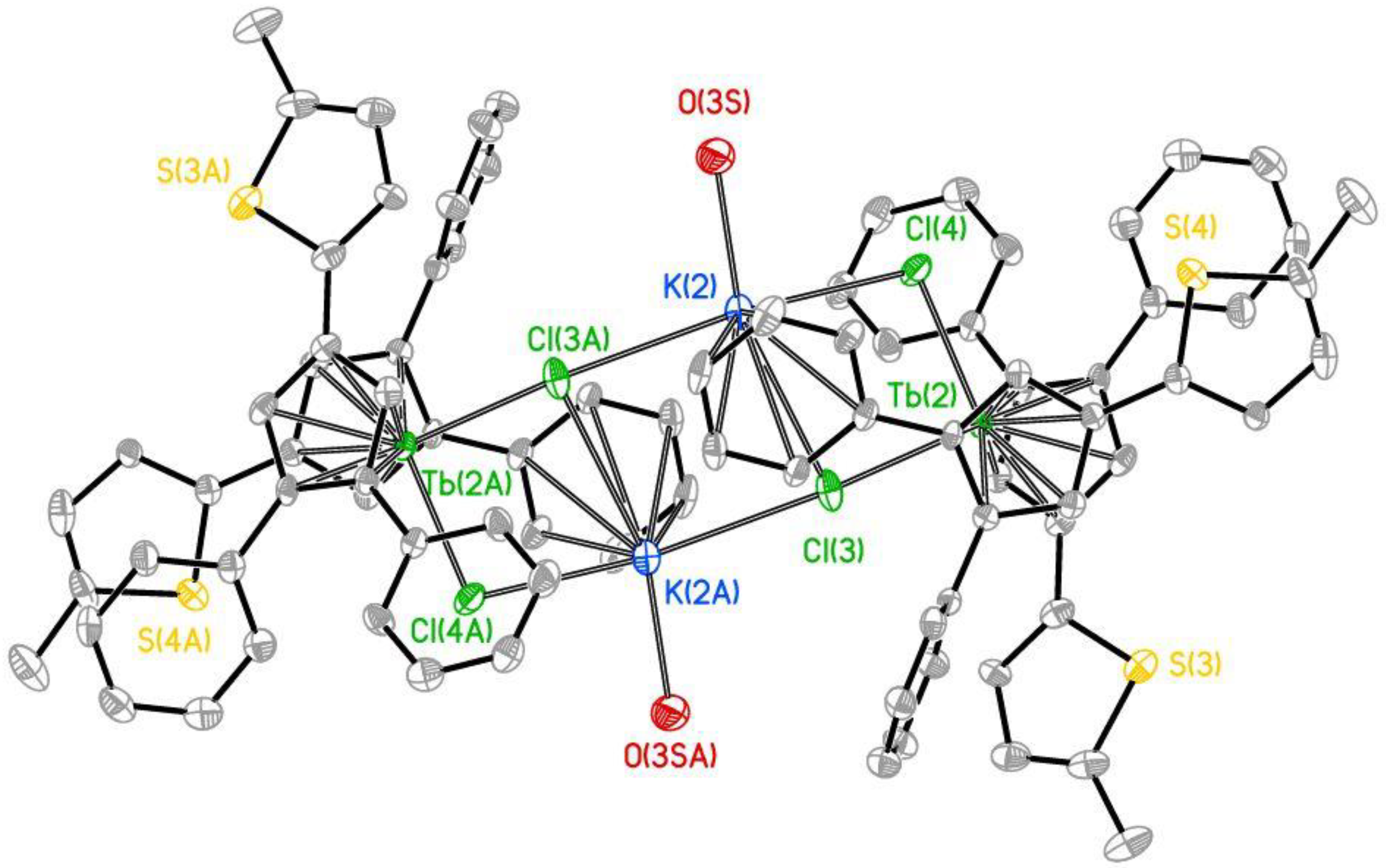
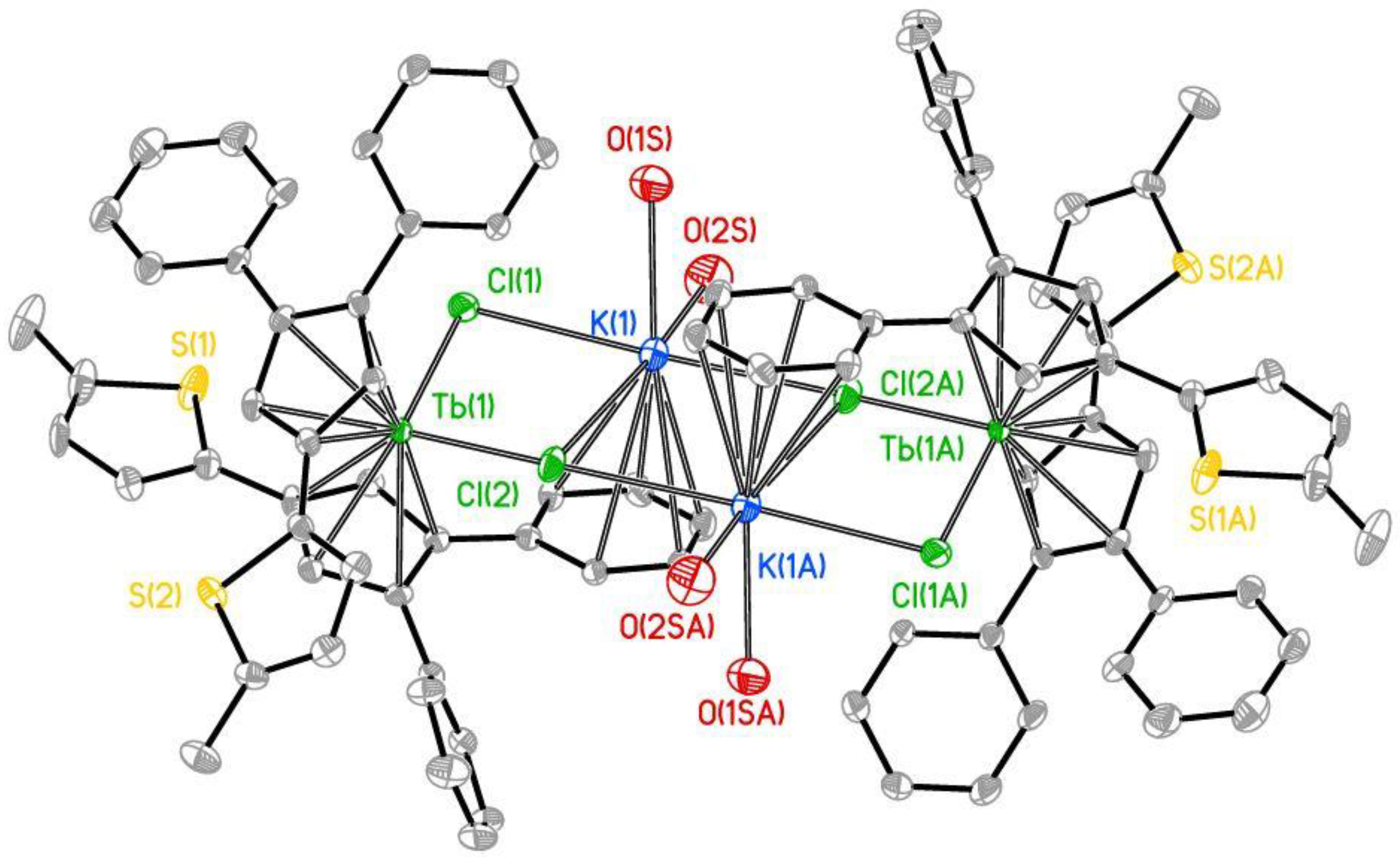
| Title 1 | 3a | 3b |
|---|---|---|
| Tb-Cpcent (Å) | 2.406(6), 2.417(6) | 2.407(6) 2.415(6) |
| Tb-Cl (Å) | 2.5895(11), 2.6322(11) | 2.6022(12), 2.6232(11) |
| K-Cl (Å) | 3.0229(16), 3.1403(15) 3.4307(17) | 3.0092(16), 3.0481(15) 3.4301(17) |
| K-O (Å) | 2.662(5), 2.759(4) | 2.638(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradov, A.A.; Komarov, P.D.; Puntus, L.N.; Lyssenko, K.A.; Nifant’ev, I.E.; Varaksina, E.A.; Taydakov, I.V.; Roitershtein, D.M. Bis(3,4-diphenyl)(2-methythienyl)cyclopentadienyl Terbium Chloride. Molbank 2022, 2022, M1517. https://doi.org/10.3390/M1517
Vinogradov AA, Komarov PD, Puntus LN, Lyssenko KA, Nifant’ev IE, Varaksina EA, Taydakov IV, Roitershtein DM. Bis(3,4-diphenyl)(2-methythienyl)cyclopentadienyl Terbium Chloride. Molbank. 2022; 2022(4):M1517. https://doi.org/10.3390/M1517
Chicago/Turabian StyleVinogradov, Alexander A., Pavel D. Komarov, Lada N. Puntus, Konstantin A. Lyssenko, Ilya E. Nifant’ev, Evgenia A. Varaksina, Ilya V. Taydakov, and Dmitrii M. Roitershtein. 2022. "Bis(3,4-diphenyl)(2-methythienyl)cyclopentadienyl Terbium Chloride" Molbank 2022, no. 4: M1517. https://doi.org/10.3390/M1517
APA StyleVinogradov, A. A., Komarov, P. D., Puntus, L. N., Lyssenko, K. A., Nifant’ev, I. E., Varaksina, E. A., Taydakov, I. V., & Roitershtein, D. M. (2022). Bis(3,4-diphenyl)(2-methythienyl)cyclopentadienyl Terbium Chloride. Molbank, 2022(4), M1517. https://doi.org/10.3390/M1517







