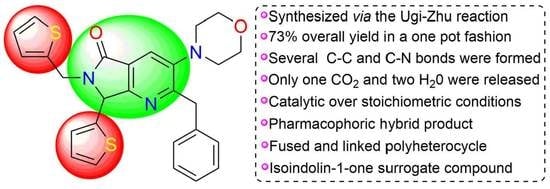
2-Benzyl-3-morpholino-7-(thiophen-2-yl)-6-(thiophen-2-ylmethyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one
Abstract
:1. Introduction
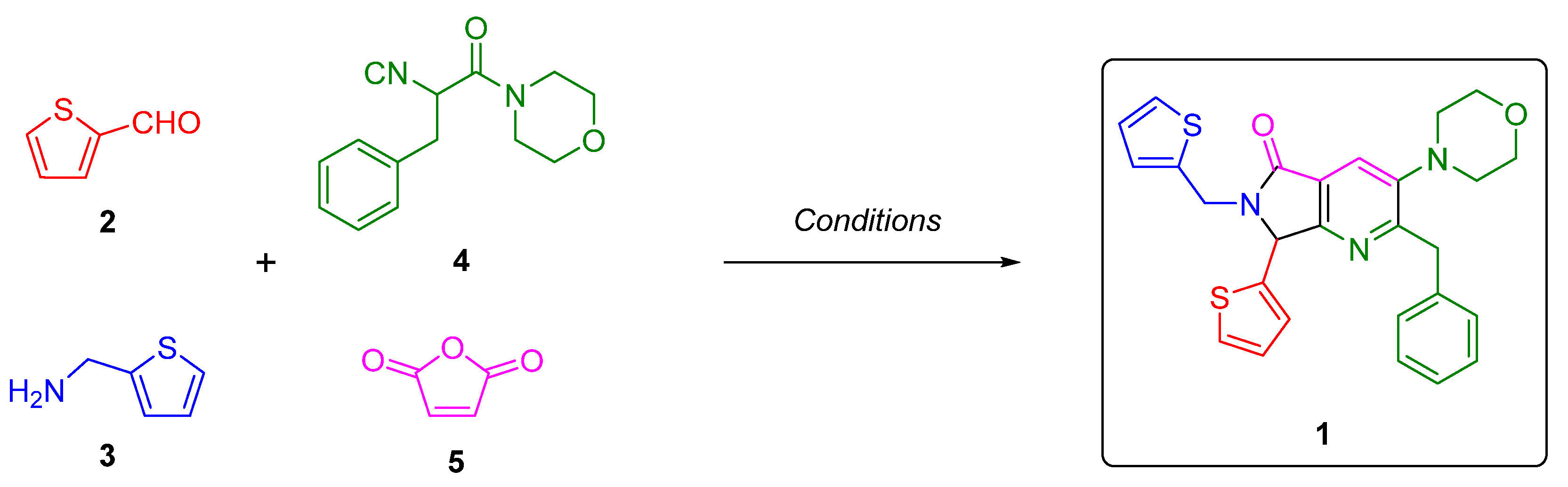
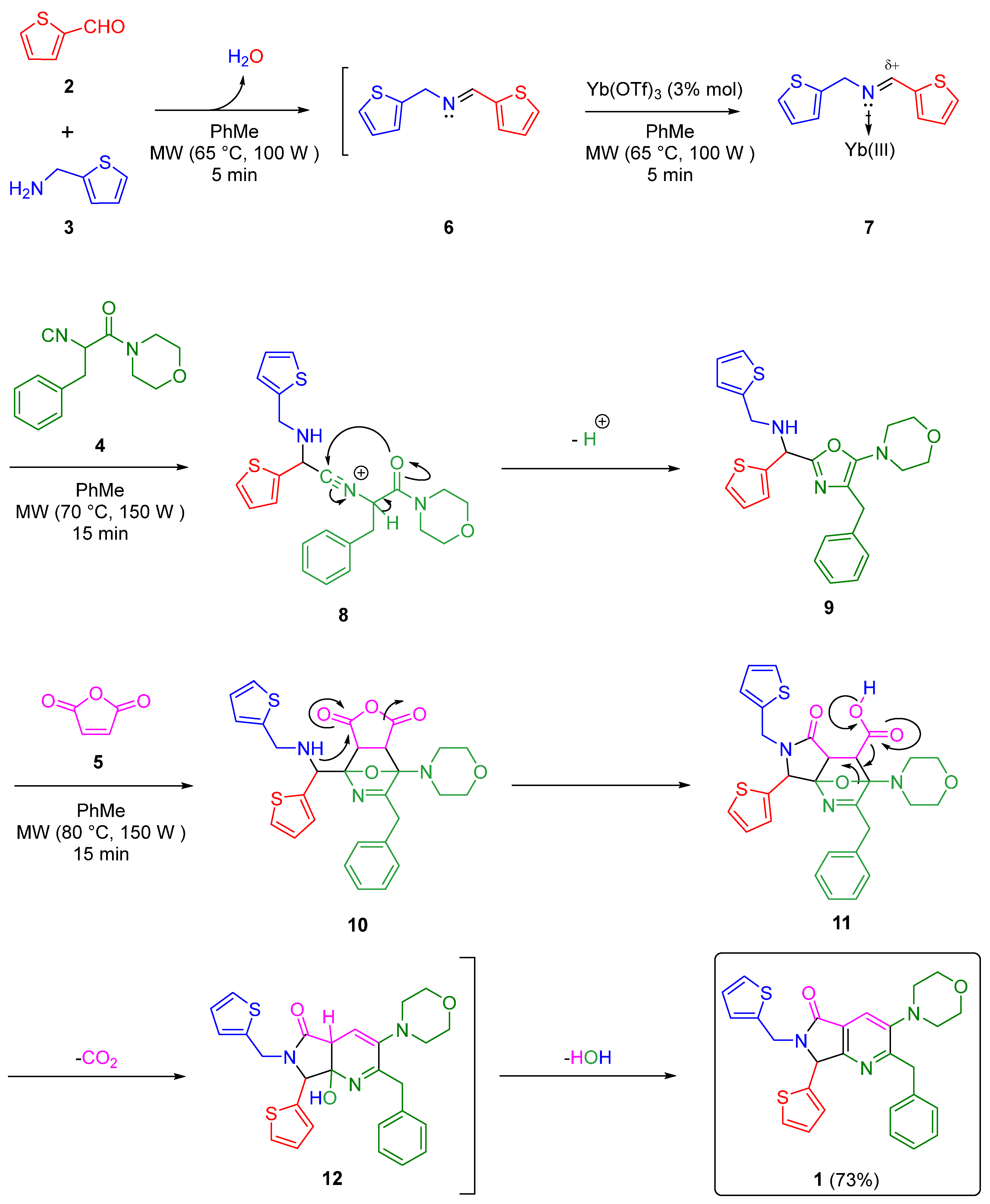
2. Results and Discussion
3. Materials and Methods
3.1. General Information, Instrumentation, Software, and Chemicals
3.2. Synthesis and Characterization of 2-Benzyl-3-morpholino-7-(thiophen-2-yl)-6-(thiophen-2-ylmethyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, S.E.; Gulati, S.; Shankaraiah, N. Recent advances in multi-component reactions and their mechanistic insights: A triennium review. Org. Chem. Front. 2021, 8, 4237–4287. [Google Scholar] [CrossRef]
- Graebin, C.S.; Ribeiro, F.V.; Rogério, K.R.; Kummerle, A.E. Multicomponent reactions for the synthesis of bioactive compounds: A review. Curr. Org. Chem. 2019, 16, 855–899. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.A.; Islas-Jácome, A.; González-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef]
- Pharande, S.G. Synthesis of lactams via isocyanide-based multicomponent reactions. Synthesis 2021, 53, 418–446. [Google Scholar] [CrossRef]
- Ruijter, E.; Orru, R.V.A. Multicomponent reactions–opportunities for the pharmaceutical industry. Drug Discov. Today Technol. 2013, 10, e15–e20. [Google Scholar] [CrossRef]
- Devasthale, P.; Wang, Y.; Wang, W.; Fevig, J.; Feng, J.X.; Wang, A.; Harrity, T.; Egan, D.; Morgan, N.; Cap, M.; et al. Optimization of Activity, Selectivity, and Liability Profiles in 5-Oxopyrrolopyridine DPP4 Inhibitors Leading to Clinical Candidate (Sa)-2-(3-(Aminomethyl)-4-(2,4-dichlorophenyl)-2-methyl-5-oxo-5H-pyrrolo[3,4-b]pyridin-6(7H)-yl) N,N-dimethylacetamide (BMS-767778). J. Med. Chem. 2013, 56, 7343–7357. [Google Scholar] [PubMed]
- Wang, Y.; Wang, H.; Jiang, X.; Jiang, Z.; Guo, T.; Ji, X.; Li, Y.; Li, Y.; Li, Z. Synthesis and broad antiviral activity of novel 2-aryl-isoindolin-1-ones towards diverse enterovirus A71 clinical isolates. Molecules 2019, 24, 985. [Google Scholar] [CrossRef] [Green Version]
- Sovic, I.; Jambon, S.; Kraljevic-Pavelic, S.; Markova-Car, E.; Ilic, N.; Depauw, S.; David-Cordonnier, M.-H.; Karminski-Zamola, G. Synthesis, antitumor activity and DNA binding features of benzothiazolyl and benzimidazolyl substituted isoindolines. Bioorg. Med. Chem. 2018, 26, 1950–1960. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Kaal, N.A.; Alterary, S.; Mubarak, M.S.; Alsayari, A.; Muhsinahd, A.B. New tiophene derivatives as antimicrobial agents. J. Heterocycl. Chem. 2019, 56, 2945–2953. [Google Scholar] [CrossRef]
- Hafez, H.N.; El-Gazzar, A.B.A. Design and synthesis of 3-pyrazolyl-thiophene, thieno[2,3-d]pyrimidines as new bioactive and pharmacological activities. Bioorg. Med. Chem. Lett. 2008, 18, 5222–5227. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wei, X.; Xu, H. Photoactivated insecticidal thiophene derivatives from Xanthopappus subacaulis. J. Nat. Prod. 2006, 69, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Liu, L.; Jiang, H.; Wu, T.; Cui, H.; Zhang, Y.; Gao, Z.; Sun, Y.; Qin, Q.; Zhao, L.; et al. Design, synthesis, and evaluation of novel 3-thiophene derivatives as potent fungistatic and fungicidal reagents based on a conformational restriction strategy. Eur. J. Med. Chem. 2022, 233, 114195. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, J.; Rathinasamy, K. Cytotoxic mechanism of tioconazole involves cell cycle arrest at mitosis through inhibition of microtubule assembly. Cytotechnology 2022, 74, 141–162. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Malinin, A.I.; Callahan, K.P.; Serebruany, V.L.; O’Connor, C.M. Effect of loading with Clopidogrel at the time of coronary stenting on platelet aggregation and glycoprotein IIb/IIIa expression and platelet-leukocyte aggregate formation. Am. J. Cardiol. 2002, 90, 312–315. [Google Scholar] [CrossRef]
- Nomura, S.; Sakamaki, S.; Hongu, M.; Kawanishi, E.; Koga, Y.; Sakamoto, T.; Yamamoto, Y.; Ueta, K.; Kimata, H.; Nakayama, K.; et al. Discovery of Canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mllitus. J. Med. Chem. 2010, 53, 6355–6360. [Google Scholar] [CrossRef] [PubMed]
- Buchstaller, H.-P.; Siebert, C.D.; Steinmetz, R.; Frank, I.; Berger, M.L.; Gottschlich, R.; Leibrock, J.; Krug, M.; Steinhilber, D.; Noe, C.R. Synthesis of thieno[2,3-b]pyridinones acting as cytoprotectants and as inhibitors of [3H]glycine binding to the N-methyl-D-aspartate (NMDA) receptor. J. Med. Chem. 2006, 49, 864–871. [Google Scholar] [CrossRef]
- Fayol, A.; Housseman, C.; Sun, X.; Janvier, P.; Bienaymé, H.; Zhu, J. Synthesis of α-Isocyano-α-alkyl(aryl)acetamides and their use in the multicomponent synthesis of 5-aminooxazole, pyrrolo[3,4-b]pyridin-5-one and 4,5,6,7-tetrahydrofuro[2,3-c]pyridine. Synthesis 2005, 1, 161–165. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; Cárdenas-Galindo, L.E.; Jerezano, A.V.; Tamariz, J.; González-Zamora, E.; Gámez-Montaño, R. Synthesis of nuevamine aza-analogues by a sequence: I-MCR–Aza-Diels–Alder–Pictet–Spengler. Synlett 2012, 23, 2951–2956. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; Gutiérrez-Carrillo, A.; García-Garibay, M.A.; González-Zamora, E. One-pot synthesis of nuevamine aza-analogues by combined use of an oxidative Ugi type reaction and aza-Diels–Alder cycloaddition. Synlett 2014, 25, 0403–0406. [Google Scholar] [CrossRef]
- Flores-Reyes, J.C.; Islas-Jácome, A.; González-Zamora, E. The Ugi three-component reaction and its variants. Org. Chem. Front. 2021, 8, 5460–5514. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W.W.-L. Rare-earth metal triflates in organic synthesis. Chem. Rev. 2002, 102, 2227–2302. [Google Scholar] [CrossRef]

| Entry a | Solvent | Catalyst b | Yield (%) |
|---|---|---|---|
| 1 | MeOH | Sc(OTf)3 | 30 |
| 2 | EtOH | Sc(OTf)3 | 17 |
| 3 | CH3CN | Sc(OTf)3 | traces |
| 4 | PhMe | Sc(OTf)3 | 60 |
| 5 | PhMe | NH4Cl | 24 |
| 6 | PhMe | Yb(OTf)3 | 73 |
| 7 | PhMe | InCl3 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Salazar, I.; Rincón-Guevara, M.A.; González-Zamora, E.; Islas-Jácome, A. 2-Benzyl-3-morpholino-7-(thiophen-2-yl)-6-(thiophen-2-ylmethyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one. Molbank 2022, 2022, M1503. https://doi.org/10.3390/M1503
Morales-Salazar I, Rincón-Guevara MA, González-Zamora E, Islas-Jácome A. 2-Benzyl-3-morpholino-7-(thiophen-2-yl)-6-(thiophen-2-ylmethyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one. Molbank. 2022; 2022(4):M1503. https://doi.org/10.3390/M1503
Chicago/Turabian StyleMorales-Salazar, Ivette, Mónica A. Rincón-Guevara, Eduardo González-Zamora, and Alejandro Islas-Jácome. 2022. "2-Benzyl-3-morpholino-7-(thiophen-2-yl)-6-(thiophen-2-ylmethyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one" Molbank 2022, no. 4: M1503. https://doi.org/10.3390/M1503
APA StyleMorales-Salazar, I., Rincón-Guevara, M. A., González-Zamora, E., & Islas-Jácome, A. (2022). 2-Benzyl-3-morpholino-7-(thiophen-2-yl)-6-(thiophen-2-ylmethyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one. Molbank, 2022(4), M1503. https://doi.org/10.3390/M1503