Abstract
(E)-3-(2-(4-methylthiazol-2-yl)hydrazineylidene)chromane-2,4-dione was synthesized for the first time and the compound was characterized by 1H and 13C spectroscopy, IR spectroscopy, and UV-Vis. The chemical structure and isomeric configuration of the molecule were confirmed by single-crystal X-ray diffraction.
1. Introduction
Due to their dominance in natural product chemistry, their large variety of pharmacological activities, and optical properties, coumarins are of crucial relevance in medicinal chemistry [,,,,]. The 2-aminothiazole derivatives of 4-hydroxycoumarin have demonstrated significant biological activity, with evidence that introduction of methyl groups on the thiazole ring results in an increase in the anticancer activity of the species []. Here we report the synthesis of the (E)-3-(2-(4-methylthiazol-2-yl)hydrazineylidene)chromane-2,4-dione isomer (3, Figure 1) as the last elusive isomer of the previously tested compounds []. Molecule 3 is further envisaged as a ligand for the preparation of antibiotic and anticancer metal complexes [].
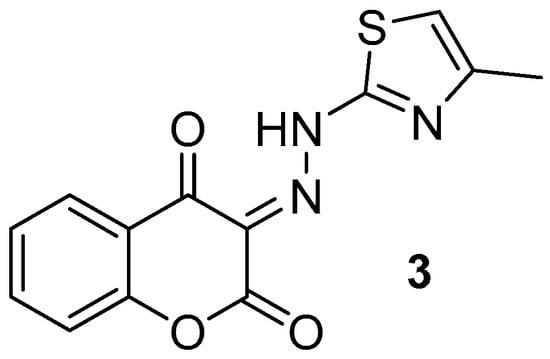
Figure 1.
Chemical structure of (E)-3-(2-(4-methylthiazol-2-yl)hydrazineylidene)chromane-2,4-dione (3).
2. Results and Discussion
2.1. Synthesis of 3
Compound 3 was prepared by coupling 4-methylthiazole-2-diazonium (2) with 4-hydroxycoumarin dissolved in a NaOH solution, according to the synthetic procedure outlined in Scheme 1. Compound 2 was prepared in situ by diazotization of 4-methylthiazol-2-amine (1) under highly acidic conditions using concentrated H2SO4 and a NaNO2 solution (Scheme 1). Compound 3 was isolated in 16 % yield as a red powder.
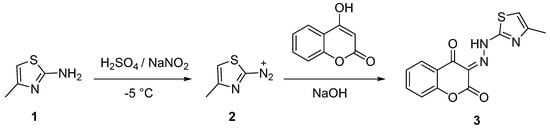
Scheme 1.
Preparation of 2-aminothiazole-4-methyl diazonium salt and its coupling with 4-hydroxycoumarin to give 3.
2.2. X-ray Crystal Structure
The 2-aminothiazole derivatives of 4-hydroxycoumarin have several tautomeric forms (Scheme 2). According to previously published gaseous phase calculation [], the diketo form (3, Scheme 2) dominates as the most stable tautomeric conformer for this type of compound. There are, however, no examples in the literature of structurally characterized isomers of this species. Therefore, it was important for us to confirm the stereochemistry of 3. Crystals of 3 suitable for X-ray analysis were grown in a dichloromethane solution layered with pentane. Figure 2 shows an ORTEP representation of the X-ray structure. The molecule crystallizes in the monoclinic P21/n space group with two asymmetric units in the unit cell. In the solid state, the molecule is planar, stabilized by intramolecular hydrogen bonding. Planes defined respectively by the thiazole and the coumarin atoms, are tilted by only 5.2°. We are aware of only one other structure which resembles our molecule, namely that of 3-[2-(5-t-butyl-1,2-oxazol-3-yl)hydrazinylidene]chroman-2,4-dione []. Unlike 3, this molecule is not planar in the solid state, but rather bent (most likely due to the presence of the bulky t-butyl). Although the same intramolecular hydrogen bonding interaction is also observed in the oxazole derivative, the planes defined respectively by the oxazole ring and the coumarin atoms form an angle of 20.3° [].
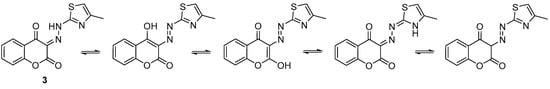
Scheme 2.
Tautomeric forms of (E)-3-(2-(4-methylthiazol-2 yl)hydrazineylidene)chromane-2,4-dione.
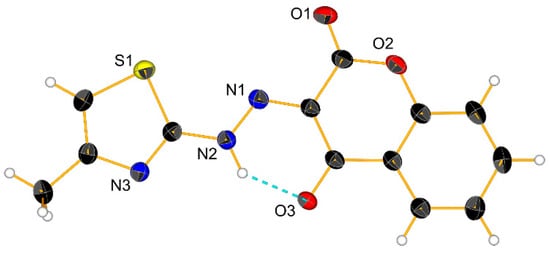
Figure 2.
Crystal structure of (E)-3-(2-(4-methylthiazol-2-yl)hydrazineylidene)chromane-2,4-dione (3). Thermal ellipsoids are at 30% probability.
3. Materials and Methods
3.1. General Comments
All commercially available reagents were used without any further purification. (E)-3-(2-(4-methylthiazol-2yl)hydrazineylidene)chromane-2,4-dione was prepared following adapted procedures from the literature [,]. 1H and 13C NMR spectra were recorded on a Bruker Advance III 400 MHz. The corresponding chemical shifts were reported in ppm, referenced to residual solvent signals. IR spectra were acquired on a Bruker TENSOR II with the following parameters: 16 scans for the background, and 32 scans for the sample with a resolution of 4 cm−1 in the 4000–400 cm−1 region. UV–Vis spectra were recorded on a Jasco V730 spectrophotometer and the samples were dissolved in DCM. Spectra of the compound are given in Supplementary Materials. Single crystal diffraction data collection of the target molecule was performed on a Stoe STADIVARI diffractometer (CuKα1 (λ = 1.5406 Å)) equipped with a cryostat from Oxford Cryosystems. The structure of 3 was solved with the SHELXT structure solution program using Intrinsic Phasing and refined with the SHELXT refinement package using Least Squares minimization [,]. The crystal structure has been deposited at the Cambridge Crystallographic Data Centre. CCDC number 2213454 contains the crystallographic data for this paper.
3.2. Synthesis of 3
An amount of 10 mmol of 2-amino-4-methyl thiazole (1) was dissolved in 5 mL of H2SO4. Using an ice–salt bath, the system was cooled and maintained between −5 to 0 °C. An aqueous solution of 7 mL of NaNO2 (10 wt.) was added dropwise to the solution of 1 and mixed for one hour by a magnetic stirrer. After one hour a mixture of 4-hydroxycoumarin (10 mmol) in 10 mL of NaOH (10 wt.) was added. A black voluminous precipitate was obtained. The mixture was stirred for 1 h in the ice bath and then for 3 h at room temperature. The precipitate was then vacuum-filtered and extracted with dichloromethane. A red compound was obtained following solvent evaporation. This was finally recrystallized from a H2O/MeOH mixture. Yield: 16 %. 1H NMR (400 MHz, CD2Cl2-d2) δ ppm 2.40 (d, J = 0.73 Hz, 3 H) 6.79 (s, 1 H) 7.26–7.45 (m, 2 H) 7.73 (ddd, J = 8.47, 7.18, 1.71 Hz, 1 H) 8.10 (d, J = 7.70 Hz, 1 H). 13C NMR (101 MHz, CD2Cl2-d2) δ ppm 17.62 (s, 1 C) 107.14 (s, 1 C) 112.23 (s, 1 C) 118.25 (s, 1 C) 120.57 (s, 1 C) 125.52 (s, 1 C) 127.76 (s, 1 C) 137.74 (s, 1 C) 152.34 (s, 1 C) 155.23 (s, 1 C) 164.58 (s, 1 C) 173.81 (s, 1 C) 179.54 (s, 1 C). IR spectra (cm−1) = 3450–3500 cm−1 (N-H), 1740 cm−1 (C=O) 1605 (C=C stretch aromatic). UV-Vis (CH2Cl2) (λmax/nm) = 260, 350, and 450. Crystals of 3 suitable for X-ray analysis were grown in a dichloromethane solution layered with pentane.
4. Conclusions
In this work, we have described the synthesis and characterization of the new compound (E)-3-(2-(4-methylthiazol-2yl)hydrazineylidene)chromane-2,4-dione, which was obtained by the diazo coupling reaction of 2-amino-4-methyl thiazole and 4-hydroxy coumarin.
Supplementary Materials
The following supporting information associated with this article can be found in the online version: 1H-NMR, 13C-NMR, IR, UV-Vis spectra, and crystallographic details of 3.
Author Contributions
Conceptualization, F.R. and F.Z.; methodology, F.R.; validation, F.Z.; formal analysis, F.R. and A.C.; investigation, F.R.; resources, F.Z.; data curation, F.R. and A.C.; writing—original draft preparation, F.R.; writing—review and editing, F.R., A.C. and F.Z.; supervision, F.Z.; project administration, F.Z.; funding acquisition, F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Swiss Federal Commission for Scholarships for Foreign Students (FCS) and University of Fribourg, Switzerland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akchurin, I.O.; Yakhutina, A.I.; Bochkov, A.Y.; Solovjova, N.P.; Medvedev, M.G.; Traven, V.F. Novel push-pull fluorescent dyes–7-(diethylamino)furo- and thieno[3,2-c]coumarins derivatives: Structure, electronic spectra and TD-DFT study. J. Mol. Struct. 2018, 1160, 215–221. [Google Scholar] [CrossRef]
- Erdogdu, Y.; Baskose, U.C.; Saglam, S.; Erdogdu, M.; Ogutcu, H.; Özçelik, S. Structural, thermal, spectroscopic, electronic and biological activity properties of coumarin-153 dyes for DSSCs: A DFT benchmark study. J. Mol. Struct. 2020, 1221, 128873. [Google Scholar] [CrossRef]
- Nagaraja, O.; Bodke, Y.D.; Kenchappa, R.; Ravi Kumar, S. Synthesis and characterization of 3-[3-(1H-benzimidazol-2-ylsulfanyl)-3-phenyl propanoyl]-2H-chromen-2-one derivatives as potential biological agents. Chem. Data Collect 2020, 27, 100369. [Google Scholar] [CrossRef]
- Nagaraja, O.; Bodke, Y.D.; Pushpavathi, I.; Ravi Kumar, S. Synthesis, characterization and biological investigations of potentially bioactive heterocyclic compounds containing 4-hydroxy coumarin. Heliyon 2020, 6, e04245. [Google Scholar] [CrossRef] [PubMed]
- Panitsiri, A.; Tongkhan, S.; Radchatawedchakoon, W.; Sakee, U. Synthesis and anion recognition studies of novel bis (4-hydroxycoumarin) methane azo dyes. J. Mol. Struct. 2016, 1107, 14–18. [Google Scholar] [CrossRef]
- Jashari, A.; Imeri, F.; Ballazhi, L.; Shabani, A.; Mikhova, B.; Dräger, G.; Popovski, E.; Huwiler, A. Synthesis and cellular characterization of novel isoxazolo- and thiazolohydrazinylidene-chroman-2,4-diones on cancer and non-cancer cell growth and death. Bioorg. Med. Chem. 2014, 22, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Sovari, S.N.; Vojnovic, S.; Bogojevic, S.S.; Crochet, A.; Pavic, A.; Nikodinovic-Runic, J.; Zobi, F. Design, synthesis and in vivo evaluation of 3-arylcoumarin derivatives of rhenium(I) tricarbonyl complexes as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 205, 112533. [Google Scholar] [CrossRef] [PubMed]
- Stamboliyska, B.; Jashari, A.; Yancheva, D.; Mikhova, B.; Batovska, D.; Popovski, E.; Mladenovska, K. Structure and radical scavenging activity of isoxazolo- and thiazolohydrazinylidenechroman-2,4-diones. Bulg. Chem. Commun. 2017, 49, 99–105. [Google Scholar]
- Jashari, A.; Popovski, E.; Mikhova, B.; Nikolova, R.P.; Shivachev, B.L. 3-[2-(5-tert-Butyl-1,2-oxazol-3-yl)hydrazinyl-idene]chroman-2,4-dione. Acta Crystallogr. E 2013, 69, o258. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.; Bodke, Y.D.; Nagaraja, O.; Lohith, T.N.; Nagaraju, G.; Sridhar, M.A. Coumarin-Benzothiazole Based Azo Dyes: Synthesis, Characterization, Computational, Photophysical and Biological Studies. J. Mol. Struct. 2021, 1246, 131170. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).