4-Methyl-7-((2-((5-methyl-1,3,4-thiadiazol-2-yl)thio)ethyl)thio)-coumarin
Abstract
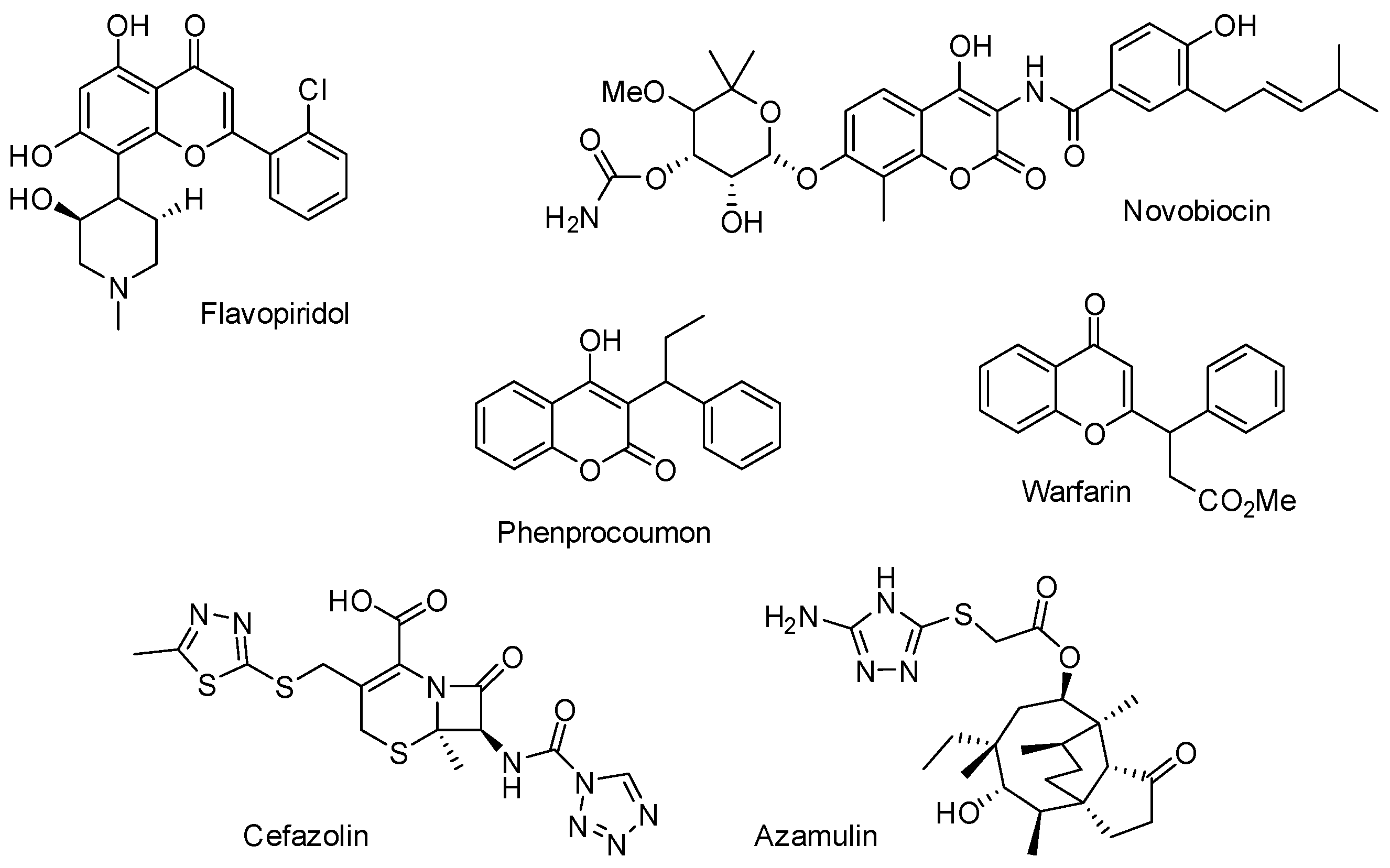
1. Introduction
2. Results and Discussion
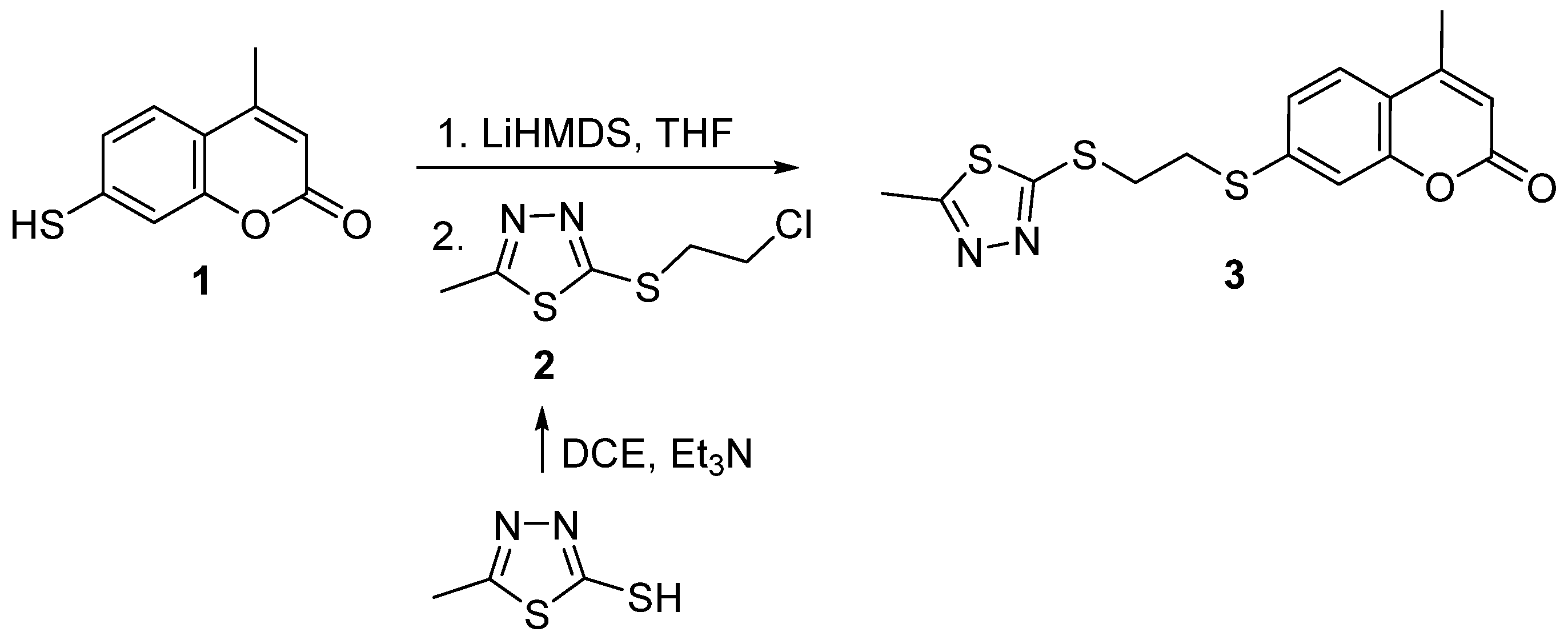
2.1. Synthesis
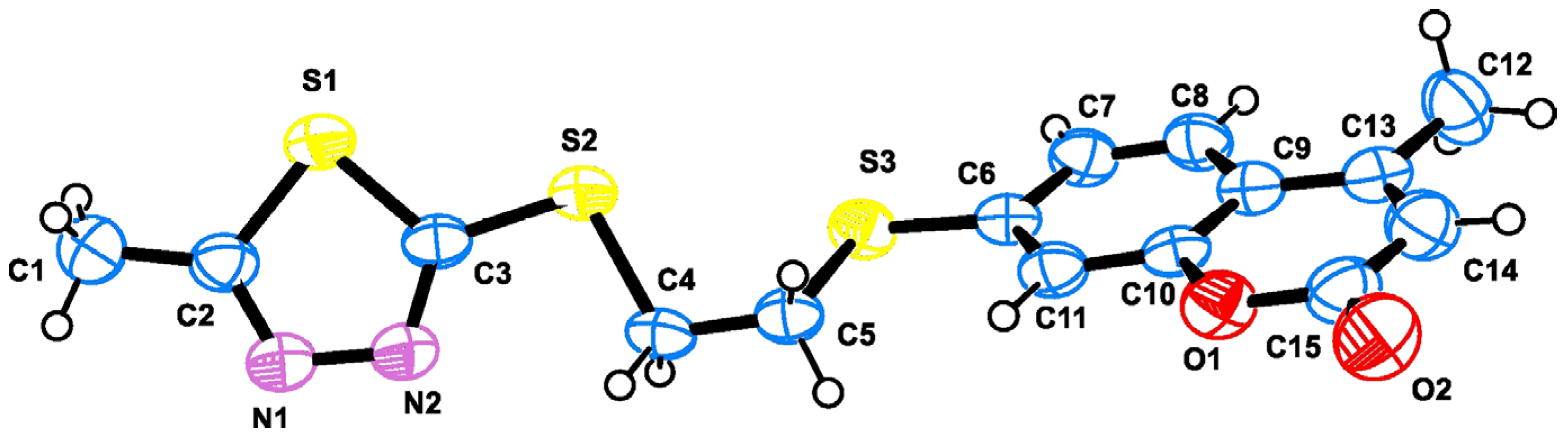
2.2. Crystallography
3. Materials and Methods
3.1. General
3.2. Synthesis of 2-((2-chloroethyl)thio)-5-methyl-1,3,4-thiadiazole
3.3. Synthesis of 4-Methyl-7-((2-((5-methyl-1,3,4-thiadiazol-2-yl)thio)ethyl)thio)-coumarin
3.4. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, G.; Zheng, H.; Guo, M.; Du, L.; Liu, G.; Wang, P. Synthesis of polymeric fluorescent brightener based on coumarin and its performances on paper as light stabilizer, Fluorescent Brightener and Surface Sizing Agent. Appl. Surf. Sci. 2016, 367, 167–173. [Google Scholar] [CrossRef]
- Oliveira, E.; Bértolo, E.; Núñez, C.; Pilla, V.; Santos, H.M.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Djafari, J.; Capelo, J.L.; Lodeiro, C. Green and red fluorescent dyes for translational applications in imaging and sensing analytes: A dual-color flag. ChemistryOpen 2017, 7, 9–52. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, T.; Sun, J.; Wang, X. Synthesis and application of coumarin fluorescence probes. RSC Adv. 2020, 10, 10826–10847. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.B.; Ihde, M.H.; Busenlehner, A.M.; Davis, D.L.; Mia, R.; Panella, J.; Fronczek, F.R.; Bonizzoni, M.; Wallace, K.J. Structural features of a family of coumarin–enamine fluorescent chemodosimeters for ion pairs. Inorg. Chem. 2021, 60, 14238–14252. [Google Scholar] [CrossRef]
- Ballin, N.Z.; Sørensen, A.T. Coumarin Content in Cinnamon Containing Food Products on the Danish Market. Food Control 2014, 38, 198–203. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Jiang, J.-G. Pharmacological and nutritional effects of natural coumarins and their structure–activity relationships. Mol. Nutr. Food Res. 2018, 62, 1701073. [Google Scholar] [CrossRef]
- Lončar, M.; Jakovljevíc, M.; Šubaríc, D.; Pavlíc, M.; Buzjak Služek, V.; Cindríc, I.; Molnar, M. Coumarins in food and methods of their determination. Foods 2020, 9, 645. [Google Scholar] [CrossRef]
- Abdallah, M.; Hijazi, A.; Graff, B.; Fouassier, J.-P.; Rodeghiero, G.; Gualandi, A.; Dumur, F.; Cozzi, P.G.; Lalevée, J. Coumarin derivatives as versatile photoinitiators for 3D printing, polymerization in water and photocomposite synthesis. Polym. Chem. 2019, 10, 872–884. [Google Scholar] [CrossRef]
- Cazin, I.; Rossegger, E.; de la Cruz, G.G.; Griesser, T.; Schlögl, S. Recent advances in functional polymers containing coumarin chromophores. Polymers 2021, 13, 56. [Google Scholar] [CrossRef]
- Lima, C.; Silva, K.; Nascimento, J.; Rossi, C.; Granato, M.; Oliveira, F. Optimization of the polyester fabric dyeing process using coumarin as a green carrier. Text. Res. J. 2022, 92, 3875–3888. [Google Scholar] [CrossRef]
- Liu, Z.; Dumur, F. Recent advances on visible light coumarin-based oxime esters as initiators of polymerization. Eur. Polym. J. 2022, 177, 111449. [Google Scholar] [CrossRef]
- Pereira, T.M.; Franco, D.P.; Vitorio, F.; Kummerle, A.E. Coumarin Compounds in Medicinal Chemistry: Some Important Examples from the Last Years. Curr. Top. Med. Chem. 2018, 18, 124–148. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending topics on coumarin and its derivatives in 2020. Molecules 2021, 26, 501. [Google Scholar] [CrossRef] [PubMed]
- Agíc, D.; Karnaš, M.; Šubaríc, D.; Lončari’c, M.; Tomíc, S.; Karačíc, Z.; Bešlo, D.; Rastija, V.; Molnar, M.; Popovíc, B.M.; et al. Coumarin derivatives act as novel inhibitors of human dipeptidyl peptidase III: Combined in vitro and in silico study. Pharmaceuticals 2021, 14, 540. [Google Scholar] [CrossRef]
- Tsivileva, O.M.; Koftin, O.V.; Evseeva, N.V. Coumarins as fungal metabolites with potential medicinal properties. Antibiotics 2022, 11, 1156. [Google Scholar] [CrossRef]
- Chaudhary, D.; Bedi, P.; Santra, S.; Pramanik, T. Synthesis and biological properties of coumarin analogue: A brief review. Lett. Org. Chem. 2022, 19, 362–387. [Google Scholar] [CrossRef]
- Nasab, N.H.; Azimian, F.; Kruger, H.G.; Kim, S.J. Coumarin-chalcones generated from 3-acetylcoumarin as a promising agent: Synthesis and pharmacological properties. ChemistrySelect 2022, 7, e202200238. [Google Scholar] [CrossRef]
- Arya, C.G.; Gondru, R.; Li, Y.; Banothu, J. Coumarin–benzimidazole hybrids: A review of developments in medicinal chemistry. Eur. J. Med. Chem. 2022, 227, 113921. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef]
- Song, X.-F.; Fan, J.; Liu, L.; Liu, X.-F.; Gao, F. Coumarin derivatives with anticancer activities: An update. Arch. Pharm. 2020, 353, 2000025. [Google Scholar] [CrossRef]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Banikazemi, Z.; Mirazimi, S.M.; Dashti, F.; Mazandaranian, M.R.; Akbari, M.; Morshedi, K.; Aslanbeigi, F.; Rashidian, A.; Chamanara, M.; Hamblin, M.R.; et al. Coumarins and gastrointestinal cancer: A new therapeutic option? Front. Oncol. 2021, 11, 752784. [Google Scholar] [CrossRef] [PubMed]
- Rubab, L.; Afroz, S.; Ahmad, S.; Hussain, S.; Nawaz, I.; Irfan, A.; Batool, F.; Kotwica-Mojzych, K.; Mojzych, M. An update on synthesis of coumarin sulfonamides as enzyme inhibitors and anticancer agents. Molecules 2022, 27, 1604. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Torres, A.; Jiménez, E.; Becerra, D.; Martínez, J.J.; Rojas, H.; Castillo, J.-C.; Macías, M.A. Synthesis, anticancer evaluation, thermal and X-ray crystallographic analysis of 2-oxo-2H-chromen-7-yl 4-chlorobenzoate using a conductively heated sealed-vessel reactor. J. Mol. Struct. 2023, 1274, 134414. [Google Scholar] [CrossRef]
- Kirsch, G.; Abdelwahab, A.B.; Chaimbault, P. Natural and synthetic coumarins with effects on inflammation. Molecules 2016, 21, 1322. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, L.C. Coumarin derivatives in inflammatory bowel disease. Molecules 2021, 26, 422. [Google Scholar] [CrossRef]
- Rostom, B.; Karaky, R.; Kassab, I.; Veitía, M.S.-I. Coumarins derivatives and inflammation: Review of their effects on the inflammatory signaling pathways. Eur. J. Pharmacol. 2022, 922, 174867. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kongot, M.; Kumar, A. Coumarin hybrid derivatives as promising leads to treat tuberculosis: Recent developments and critical aspects of structural design to exhibit anti-tubercular activity. Tuberculosis 2021, 127, 102050. [Google Scholar] [CrossRef]
- Mujeeb, S.; Singh, K.; Yogi, B.; Ansari, V.; Sinha, S. A review on coumarin derivatives as potent anti-tuberculosis agents. Mini-Rev. Med. Chem. 2022, 22, 1064–1080. [Google Scholar] [CrossRef]
- Cheke, R.S.; Patel, H.M.; Patil, V.M.; Ansari, I.A.; Ambhore, J.P.; Shinde, S.D.; Kadri, A.; Snoussi, M.; Adnan, M.; Kharkar, P.S.; et al. Molecular insights into coumarin analogues as antimicrobial agents: Recent developments in drug discovery. Antibiotics 2022, 11, 566. [Google Scholar] [CrossRef]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-Thiadiazole and its derivatives: A review on recent progress in biological activities. Chem. Biol. Drug Des. 2013, 81, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J. Biological and pharmacological activities of 1,3,4-thiadiazole based compounds. Mini Rev. Med. Chem. 2015, 15, 762–775. [Google Scholar] [CrossRef]
- Joseph, L.; George, M.; Mathews, P. A review on various biological activities of 1,3,4- thiadiazole derivatives. J. Pharm. Chem. Biol. Sci. 2015, 3, 329–345. [Google Scholar]
- Schatz, J.; Gogić, K.; Benkert, T. 1,3,4-Thiadiazoles. In Comprehensive Heterocyclic Chemistry IV, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, Chapter 5.10; pp. 407–447. [Google Scholar] [CrossRef]
- Aliabadi, A. 1,3,4-Thiadiazole based anticancer agents. Anti-Cancer Agents Med. Chem. 2016, 16, 1301–1314. [Google Scholar] [CrossRef]
- Raj, V.; Rai, A.; Saha, S. Human Cancer cell line based approach of 1,3,4-thiadiazole and its fused ring: A comprehensive review. Anti-Cancer Agents Med. Chem. 2017, 17, 500–523. [Google Scholar] [CrossRef]
- Janowska, S.; Paneth, A.; Wujec, M. Cytotoxic properties of 1,3,4-Thiadiazole derivatives—A review. Molecules 2020, 25, 4309. [Google Scholar] [CrossRef]
- Obakachi, V.A.; Kushwaha, B.; Kushwaha, N.D.; Mokoena, S.; Ganai, A.M.; Pathan, T.K.; van Zyl, W.E.; Karpoormath, R. Synthetic and anti-cancer activity aspects of 1, 3, 4-thiadiazole containing bioactive molecules: A concise review. J. Sulfur Chem. 2021, 42, 670–691. [Google Scholar] [CrossRef]
- Serban, G.; Stanasel, O.; Serban, E.; Bota, S. 2-Amino-1,3,4-thiadiazole as a potential scaffold for promising antimicrobial agents. Drug Des. Devel. Ther. 2018, 12, 1545–1566. [Google Scholar] [CrossRef]
- Barbosa, G.A.D.; de Aguiar, A.P. Synthesis of 1,3,4-thiadiazole derivatives and microbiological activities: A review. Rev. Virtual Quim. 2019, 11, 806–848. [Google Scholar] [CrossRef]
- Anthwal, T.; Nain, S. 1,3,4-Thiadiazole scaffold: As anti-epileptic agents. Front. Chem. 2022, 9, 671212. [Google Scholar] [CrossRef] [PubMed]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kamiński, D.; Creaven, B.; Jenkins, H.; Radomski, P.; Miłoś, A.; Ślusarczyk, L.; et al. Novel coumarin-thiadiazole hybrids and their Cu(II) and Zn(II) complexes as potential antimicrobial agents and acetylcholinesterase. Int. J. Mol. Sci. 2021, 22, 9709. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules 2011, 16, 6833–6843. [Google Scholar] [CrossRef] [PubMed]
- Sabt, A.; Abdelrahman, M.T.; Abdelraof, M.; Rashdan, H.R.M. Investigation of novel mucorales fungal inhibitors: Synthesis, in-silico study and anti-fungal potency of novel class of coumarin-6-sulfonamides-thiazole and thiadiazole hybrids. ChemistrySelect 2022, 7, e202200691. [Google Scholar] [CrossRef]
- Morsy, S.A.; Farahat, A.A.; Nasr, M.N.A.; Tantawy, A.S. Synthesis, molecular modeling and anticancer activity of new coumarin containing compounds. Saudi Pharm. J. 2017, 25, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Toan, V.N.; Thanh, N.D.; Tri, N.M. 1,3,4-Thiadiazoline−coumarin hybrid compounds containing D-glucose/D-galactose moieties: Synthesis and evaluation of their antiproliferative activity. Arabian J. Chem. 2021, 14, 103053. [Google Scholar] [CrossRef]
- Ujan, R.; Saeed, A.; Channar, P.A.; Larik, F.A.; Abbas, Q.; Alajmi, M.F.; El-Seedi, H.R.; Rind, M.A.; Hassan, M.; Raza, H.; et al. Drug-1,3,4-thiadiazole conjugates as novel mixed-type inhibitors of acetylcholinesterase: Synthesis, molecular docking, pharmacokinetics, and ADMET evaluation. Molecules 2019, 24, 860. [Google Scholar] [CrossRef]
- Blouin, M.; Grimm, E.L.; Gareau, Y.; Gagnon, M.; Juteau, H.; Laliberte, S.; Mackay, B.; Friesen, R. Thiadiazole substituted Coumarin Derivatives and their Use as Leukotriene Biosynthesis Inhibitor. Patent WO200609, 28 September 2006. [Google Scholar]
- Bruker AXS Inc. APEX 3; Bruker Advanced X-ray Solutions: Madison, WI, USA, 2016. [Google Scholar]
- Bruker AXS Inc. Saint and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurteva, V.; Rusew, R.; Shivachev, B. 4-Methyl-7-((2-((5-methyl-1,3,4-thiadiazol-2-yl)thio)ethyl)thio)-coumarin. Molbank 2022, 2022, M1491. https://doi.org/10.3390/M1491
Kurteva V, Rusew R, Shivachev B. 4-Methyl-7-((2-((5-methyl-1,3,4-thiadiazol-2-yl)thio)ethyl)thio)-coumarin. Molbank. 2022; 2022(4):M1491. https://doi.org/10.3390/M1491
Chicago/Turabian StyleKurteva, Vanya, Rusi Rusew, and Boris Shivachev. 2022. "4-Methyl-7-((2-((5-methyl-1,3,4-thiadiazol-2-yl)thio)ethyl)thio)-coumarin" Molbank 2022, no. 4: M1491. https://doi.org/10.3390/M1491
APA StyleKurteva, V., Rusew, R., & Shivachev, B. (2022). 4-Methyl-7-((2-((5-methyl-1,3,4-thiadiazol-2-yl)thio)ethyl)thio)-coumarin. Molbank, 2022(4), M1491. https://doi.org/10.3390/M1491





