(η4-Tetraphenylcyclobutadiene)-(η5-pentaphenylcyclopentadienyl)-cobalt
Abstract
1. Introduction
2. Results
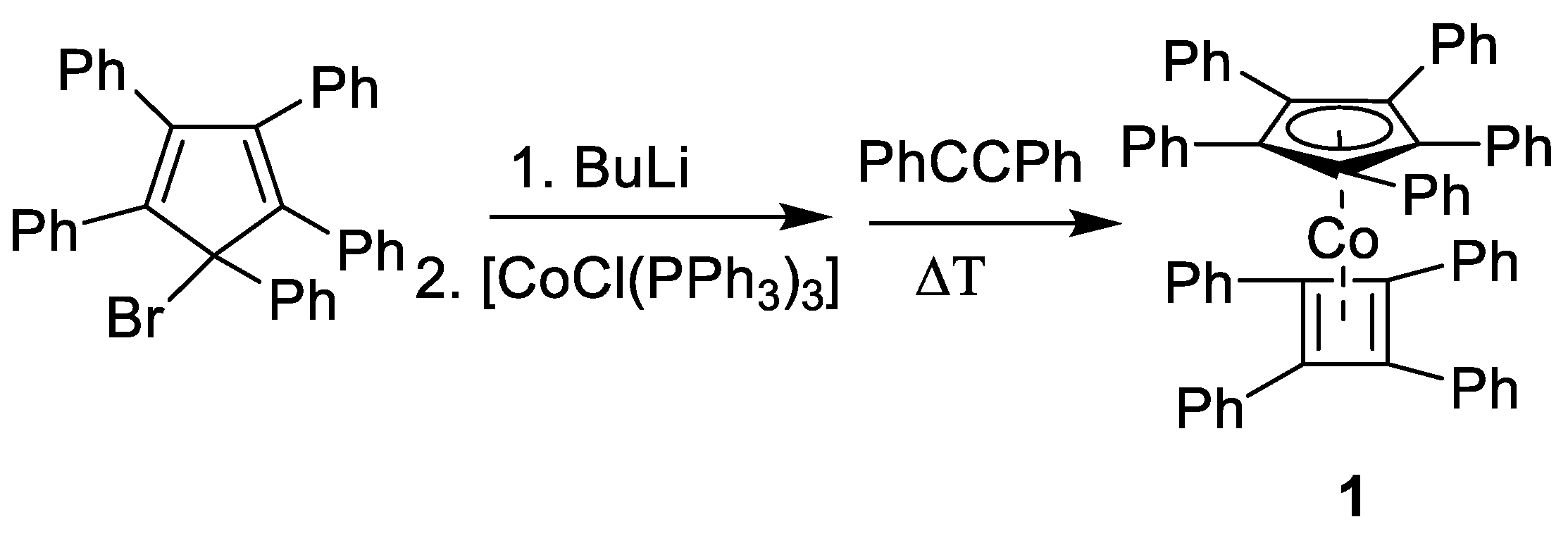
2.1. Synthesis
2.2. Spectroscopic Characterization
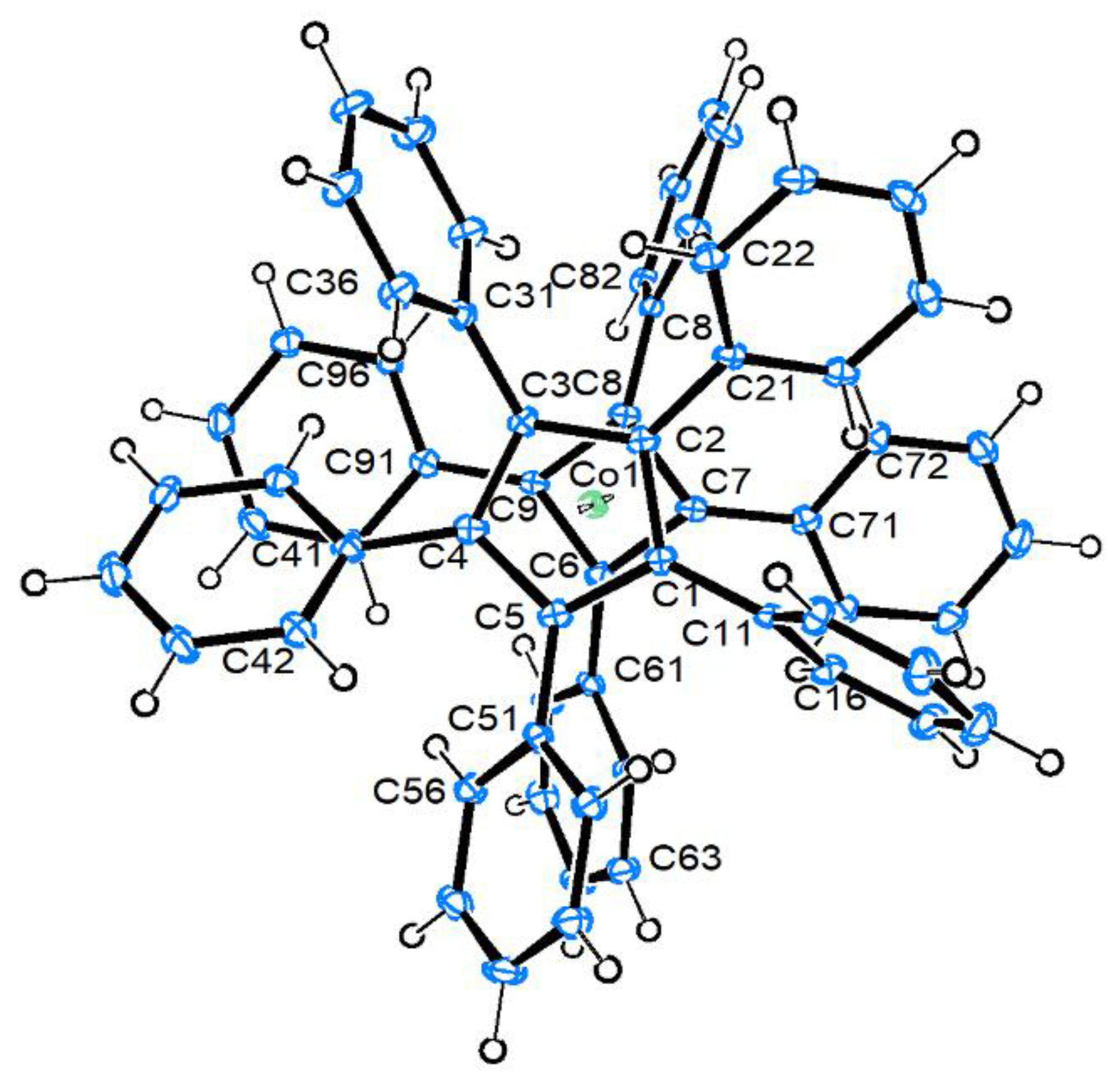
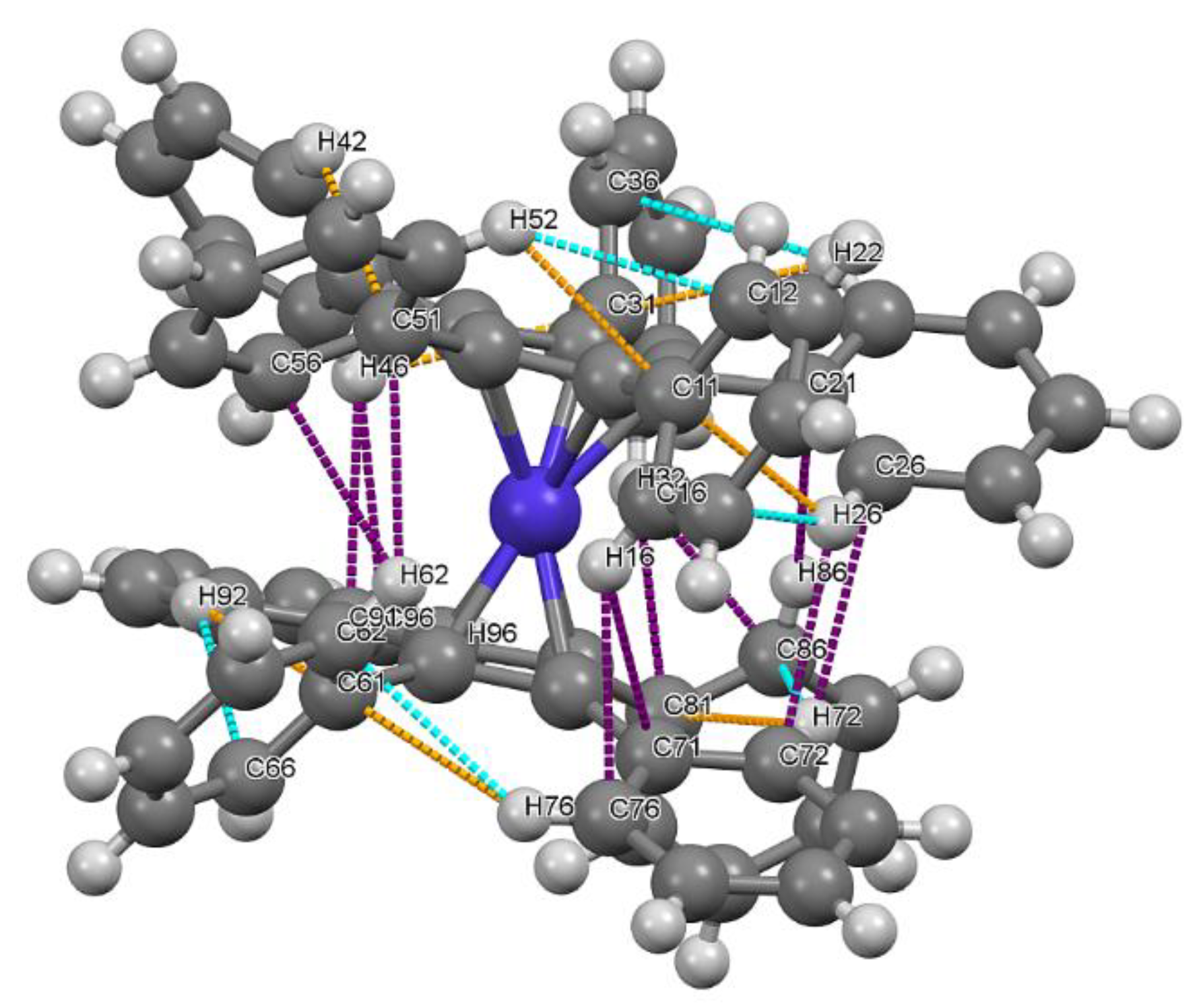
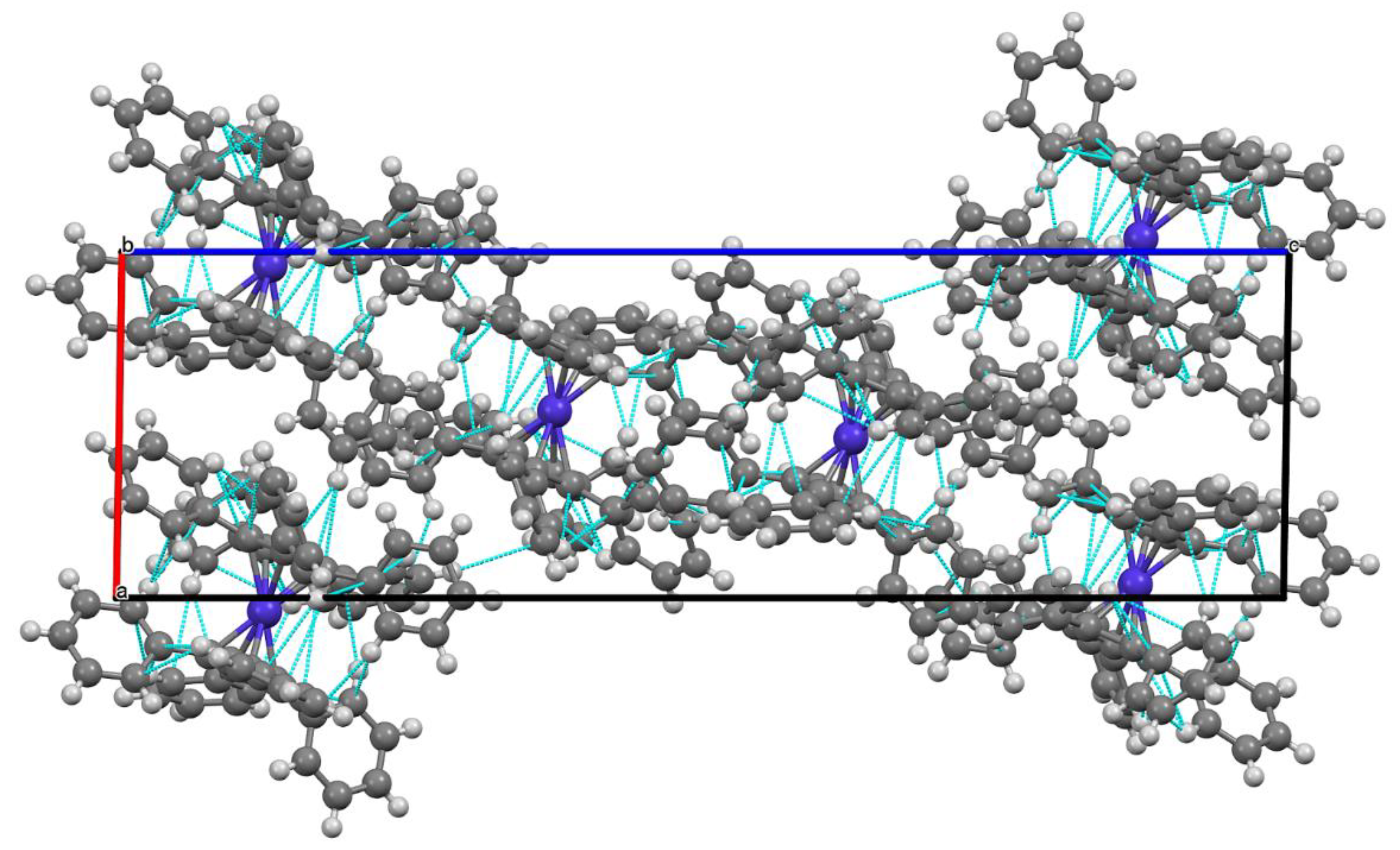
2.3. Molecular and Crystal Structure
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Crystal Structure Determinations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, L.D.; Lindall, C.M.; Masters, A.F.; Clentsmith, G.K.B. Penta-arylcyclopentadienyl complexes. Coord. Chem. Rev. 2011, 255, 1733–1790. [Google Scholar] [CrossRef]
- Schulte, Y.; Weinert, H.; Wölper, C.; Schulz, S. Direct Synthesis of Pentaarylcyclopentadienyl Sandwich Complexes of s, p, and d-Block metals. Organometallics 2020, 39, 206–216. [Google Scholar] [CrossRef]
- Chakraborty, U.; Modl, M.; Mühldorf, B.; Bodensteiner, M.; Demeshko, S.; van Velzen, N.J.C.; Harder, S.; Wolf, R. Pentaarylcyclopentadienyl Iron, Cobalt, and Nickel Halides. Inorg. Chem. 2016, 55, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbecker, D.; Harder, S.; Jansen, G. Insight in Structures of Superbulky Metallocenes with the CpBIG Ligand: Theoretical Considerations of Decaphenyl Metallocenes. Z. Anorg. Allg. Chem. 2010, 636, 2257–2261. [Google Scholar] [CrossRef]
- Ruspic, C.; Moss, J.R.; Schürmann, M.; Harder, S. Remarkable Stability of Metallocenes with Superbulky Ligands: Spontaneous Reduction of SmIII to SmII. Angew. Chem. Int. Ed. 2008, 47, 2121–2126. [Google Scholar] [CrossRef]
- Arthurs, R.A.; Richards, C.J. Multiple Acetylation of Pentaphenylferrocene—Synthesis and Asymmetric Reduction of 1-Acetyl-1′,2′,3′,4′,5′-penta(paraacetylphenyl)ferrocene. Eur. J. Inorg. Chem. 2018, 2018, 1655–1659. [Google Scholar] [CrossRef]
- Ruble, J.C.; Latham, H.A.; Fu, G.C. Effective Kinetic Resolution of Secondary Alcohols with a Planar-Chiral Analogue of 4-(Dimethylamino)pyridine. Use of the Fe(C5Ph5) Group in Asymmetric Catalysis. J. Am. Chem. Soc. 1997, 119, 1492–1493. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, J. Half-sandwich scandium dibenzyl complexes bearing penta- or tetra-arylcyclopentadienyl ligands: Synthesis, structure and syndiospecific styrene polymerization activity. New J. Chem. 2020, 44, 17333–17340. [Google Scholar] [CrossRef]
- Brydges, S.; Harrington, L.E.; McClinchey, M.J. Sterically hindered organometallics: Multi-n-rotor (n = 5, 6 and 7) molecular propellers and the search for correlated rotations. Coord. Chem. Rev. 2002, 233, 75–105. [Google Scholar] [CrossRef]
- Kottas, G.S.; Clarke, L.I.; Horinek, D.; Michl, J. Artificial Molecular Rotors. Chem. Rev. 2005, 105, 1281–1376. [Google Scholar] [CrossRef]
- Gisbert, Y.; Abid, S.; Kammerer, C.; Rapenne, G. Molecular Gears: From Solution to Surfaces. Chem. Eur. J. 2021, 27, 12019. [Google Scholar] [CrossRef] [PubMed]
- Michl, J.; Sykes, E.C.H. Molecular Rotors and Motors: Recent Advances and Future Challenges. ACS nano 2009, 3, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.M.; Richards, C.J. A Metallocene Molecular Gear. Tetrahedron Lett. 1997, 38, 7803–7808. [Google Scholar] [CrossRef]
- Uno, M.; Ando, K.; Komatsuzaki, N.; Tsuda, T.; Tanaka, T.; Sawada, M.; Takahashi, S. Synthesis and structure of planar-chiral (1,2,4-trisubstituted cyclopentadienyl)cobalt(tetraarylcyclobutadiene) complexes containing three different chiralities in one molecule. J. Organomet. Chem. 1994, 473, 303–311. [Google Scholar] [CrossRef]
- Kosaka, T.; Iwai, S.; Fukuhara, G.; Imai, Y.; Mori, T. Hydrostatic Pressure on Toroidal Interaction and Propeller Chirality of Hexaarylbenzenes: Explicit Solvent Effects on Differential Volumes in Methylcyclohexane and Hexane. Chem. Eur. J. 2019, 25, 2011–2018. [Google Scholar] [CrossRef]
- Kosaka, T.; Iwai, S.; Inoue, Y.; Moriuchi, T.; Mori, T. Solvent and Temperature Effects on Dynamics and Chiroptical Properties of Propeller Chirality and Toroidal Interaction of Hexaarylbenzenes. J. Phys. Chem. A 2018, 122, 2455–2463. [Google Scholar] [CrossRef]
- Vij, V.; Bhalla, V.; Kumar, M. Hexaarylbenzene: Evolution of Properties and Applications of Multitalented Scaffold. Chem. Rev. 2016, 116, 9565–9627. [Google Scholar] [CrossRef]
- Rios, C.; Salcedo, R. Computational Study of Electron Delocalization in Hexaarylbenzenes. Molecules 2014, 19, 3274–3296. [Google Scholar] [CrossRef]
- Lambert, C. Hexaarylbenzenes—Prospects for Toroidal Delocalization of Charge and Energy. Angew. Chem. Int. Ed. 2005, 14, 7337–7339. [Google Scholar] [CrossRef]
- Gagnon, E.; Halperin, S.D.; Metivaud, V.; Maly, K.E.; Wuest, J.D. Tampering with Molecular Cohesion in Crystals of Hexaphenylbenzenes. J. Org. Chem. 2010, 75, 399–406. [Google Scholar] [CrossRef]
- Sünkel, K.; Klein-Heßling, C. Molecular Structures oft he pentaphenylcyclopentadienyl iron complexes [(C5Ph5)Fe(CO)2R] (R = Me, Ph, iPr and Bu). Acta Cryst. C 2021, 77, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Klein-Heßling, C.; Sünkel, K. Synthesis and Characterization oft he Complete Series [(C5H5-nCln)Co(C4Ph4)] (n = 1–5). ChemistrySelect 2021, 6, 7183–7187. [Google Scholar] [CrossRef]
- Chambers, J.W.; Baskar, A.J.; Bott, S.G.; Atwood, J.L.; Rausch, M.D. Formation and Molecular Structures of η5-Pentabenzylcyclopentadienyl and η5-Pentaphenylcyclopentadienyl Dicarbonyl Derivatives of Cobalt and Rhodium. Organometallics 1986, 5, 1635–1641. [Google Scholar] [CrossRef]
- Scheer, M.; Becker, U. Dreikomponentenreaktionen unter photochemischen Bedingungen–der Transformationsweg des P4-Phosphors in der Koordinationssphäre von Co-Komplexen. J. Organomet. Chem. 1997, 545–546, 451–460. [Google Scholar] [CrossRef]
- Field, L.D.; Hambley, T.W.; He, T.; Masters, A.F.; Turner, P. The Structures of the Decaphenylmetallocenium Cations of Chromium and Cobalt. Austr. J. Chem. 1997, 50, 1035–1042. [Google Scholar] [CrossRef]
- Drobnik, S.; Stoll, C.; Nöth, H.; Polborn, K.; Hiller, W.; Lorenz, I.-P. Photolytically Induced Reactions of CpCo(CO)2 (Cp = C5H5, C5H4Me, C5Me5 and C5Ph5) with Thiirane Yielding Dinuclear 1,2-Ethanedithiolato-S,S Complexes. Z. Naturforsch. 2006, 61b, 1365–1376. [Google Scholar] [CrossRef]
- Ficker, R.; Hiller, W.; Drobnik, S.; Lorenz, I.-P. Crystal Structure of (η5-pentaphenylcyclopentadienyl)-1,2-ethen-diolatocobalt(III), C37H27CoS2. Z. Krist. 1996, 211, 845–846. [Google Scholar]
- Komatsu, H.; Yamazaki, H. Synthesis of homo- and heterodinuclear metal complexes with dimethylsilylene bridged unsymmetrical bis(cyclopentadienyl) ligand. J. Organomet. Chem. 2001, 634, 109–121. [Google Scholar] [CrossRef]
- Harrison, R.M.; Brotin, T.; Noll, B.C.; Michl, J. Toward a Square-Grid Polymer: Synthesis and Structure of Pedestal-Mounted Tetragonal Star Connectors, C4R4-Co-C5Y5. Organometallics 1997, 16, 3401–3412. [Google Scholar] [CrossRef]
- Kumar, D.; Deb, M.; Singh, J.; Keshav, K.; Elias, A.J. Chemistry oft he highly stable hindered cobalt sandwich compound η5-Cp)Co(η4-C4Ph4) and ist derivatives. Coord. Chem. Rev. 2016, 306, 115–170. [Google Scholar] [CrossRef]
- Riley, P.E.; Davis, R.E. Crystal and Molecular Structure of (π-Cyclopentadienyl)(π-Cyclobutadiene)cobalt. J. Organomet. Chem. 1976, 113, 157–166. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, S.; Yamazaki, H. Chlorotris(triphenylphosphine)cobalt. Inorg. Synth. 1989, 26, 190–191. [Google Scholar]
- Janiak, C.; Schumann, H.; Stader, C.; Wrackmeyer, B.; Zuckerman, J.J. Decaphenylgermanocen, -stannocen und –plumbocen sowie Pentaphenylstannocen: Synthese, Eigenschaften und CPMAS-Metall-NMR-Messungen. Chem. Ber. 1988, 121, 1745–1751. [Google Scholar] [CrossRef]
- Farrugia, L.J. WINGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Sheldrick, G.M. SHELXT-integrated space group and crystal structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
| Compound | Co–CTcp [Å] | Co–CTCb [Å] | Co–CCp [Å] | Co–CCb [Å] | (C–C)Cp [Å] | (C–C)Cb [Å} | CCp–Ci [Å} | CCb–Ci [Å] | αcp [°] | αcb [°] | ωcp [°] | ωcb [°] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.7170(14) | 1.7306(15) | 2.103(3) | 2.020(3) | 1.433(3) | 1.460(3) | 1.488(4) | 1.480(4) | 6.0 | 12.9 | 72.8(2) | 55.5(2) |
| 2.082(3) | 2.029(3) | 1.443(3) | 1.465(3) | 1.483(4) | 1.457(4) | 6.1 | 14.7 | 37.0(2) | 23.5(2) | |||
| 2.082(3) | 1.993(3) | 1.449(3) | 1.472(3) | 1.491(4) | 1.481(4) | 9.8 | 20.0 | 72.5(2) | 75.0(2) | |||
| 2.142(3) | 2.028(3) | 1.438(3) | 1.469(3) | 1.480(4) | 1.460(4) | 7.7 | 4.9 | 42.7(2) | 6.6(2) | |||
| 2.143(3) | 1.451(3) | 1.480(4) | 6.0 | 46.2(2) | ||||||||
| GAHRUC | 1.705 | – | 2.073(8)– | – | 1.41(1)– | – | 1.46(1)– | – | 2.0– | – | 50(1)– | – |
| 2.111(8) | 1.45(1) | 1.49(1) | 7.2 | 67(1) | ||||||||
| TUPQAW | 1.671 | – | 2.058(4)– | – | 1.430(5)– | – | 1.476(5)– | – | 0.7– | – | 39.0(6)– | – |
| 2.094(3) | 1.442(6) | 1.485(6) | 7.8 | 64.5(5) | ||||||||
| POHXAL | 1.750 | – | 2.118– | – | 1.409(9)– | – | 1.48(1)– | – | 6.8– | – | 38(1)– | – |
| 2.157 | 1.46(1) | 1.50(1) | 11.8 | 51(1) | ||||||||
| NICDIM | 1.706 | – | 2.068(9)– | – | 1.43(1)– | – | 1.48(1)– | – | 6.5– | – | 38(1)– | – |
| 2.134(8) | 1.45(1) | 1.50(2) | 12.1 | 60(1) | ||||||||
| NEVDAT | 1.685 | 1.708 | 2.06(1)– | 1.97(1)– | 1.41(2)– | 1.44(2)– | – | 1.48(1)– | – | 3.5– | – | 7(2)– |
| 2.11(1) | 2.03(1) | 1.49(2) | 1.48(2) | 1.51(1) | 7.4 | 41(2) | ||||||
| UZUQE | 1.680 | 1.709 | 2.058(4)– | 1.997(4)– | 1.408(7)– | 1.457(6)– | – | 1.461(6)– | – | 4.0– | – | 15.3(7)– |
| 2.078(6) | 2.003(5) | 1.430(6) | 1.478(5) | 1.468(6) | 8.9 | 36.0(7) | ||||||
| CPBUCO01 | 1.678 | 1.691 | 2.058(4)– | 1.982(2)– | 1.364(6)– | 1.457(4)– | – | 1.468(3)– | – | 3.9– | – | 26.6– |
| 2.078(6) | 1.989(3) | 1.386(6) | 1.473(6) | 1.470(4) | 7.3 | 35.5 | ||||||
| WEBMUL10 | 1.693 | 1.698 | 2.071(5)– | 1.986(5)– | 1.416(7)– | 1.457(7)– | 1.467(8) | 1.469(7)– | 0.7 | 4.7– | 5.1(9) | 12.4– |
| 2.098(5) | 1.990(5) | 1.438(7) | 1.473(7) | 1.475(7) | 10.0 | 40.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein-Heßling, C.; Sünkel, K. (η4-Tetraphenylcyclobutadiene)-(η5-pentaphenylcyclopentadienyl)-cobalt. Molbank 2022, 2022, M1502. https://doi.org/10.3390/M1502
Klein-Heßling C, Sünkel K. (η4-Tetraphenylcyclobutadiene)-(η5-pentaphenylcyclopentadienyl)-cobalt. Molbank. 2022; 2022(4):M1502. https://doi.org/10.3390/M1502
Chicago/Turabian StyleKlein-Heßling, Christian, and Karlheinz Sünkel. 2022. "(η4-Tetraphenylcyclobutadiene)-(η5-pentaphenylcyclopentadienyl)-cobalt" Molbank 2022, no. 4: M1502. https://doi.org/10.3390/M1502
APA StyleKlein-Heßling, C., & Sünkel, K. (2022). (η4-Tetraphenylcyclobutadiene)-(η5-pentaphenylcyclopentadienyl)-cobalt. Molbank, 2022(4), M1502. https://doi.org/10.3390/M1502







