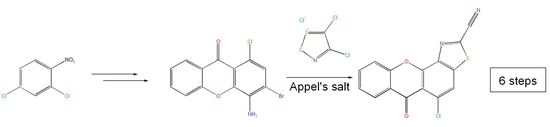
5-Chloro-6-oxo-6H-xantheno[4,3-d]thiazole-2-carbonitrile
Abstract
:1. Introduction
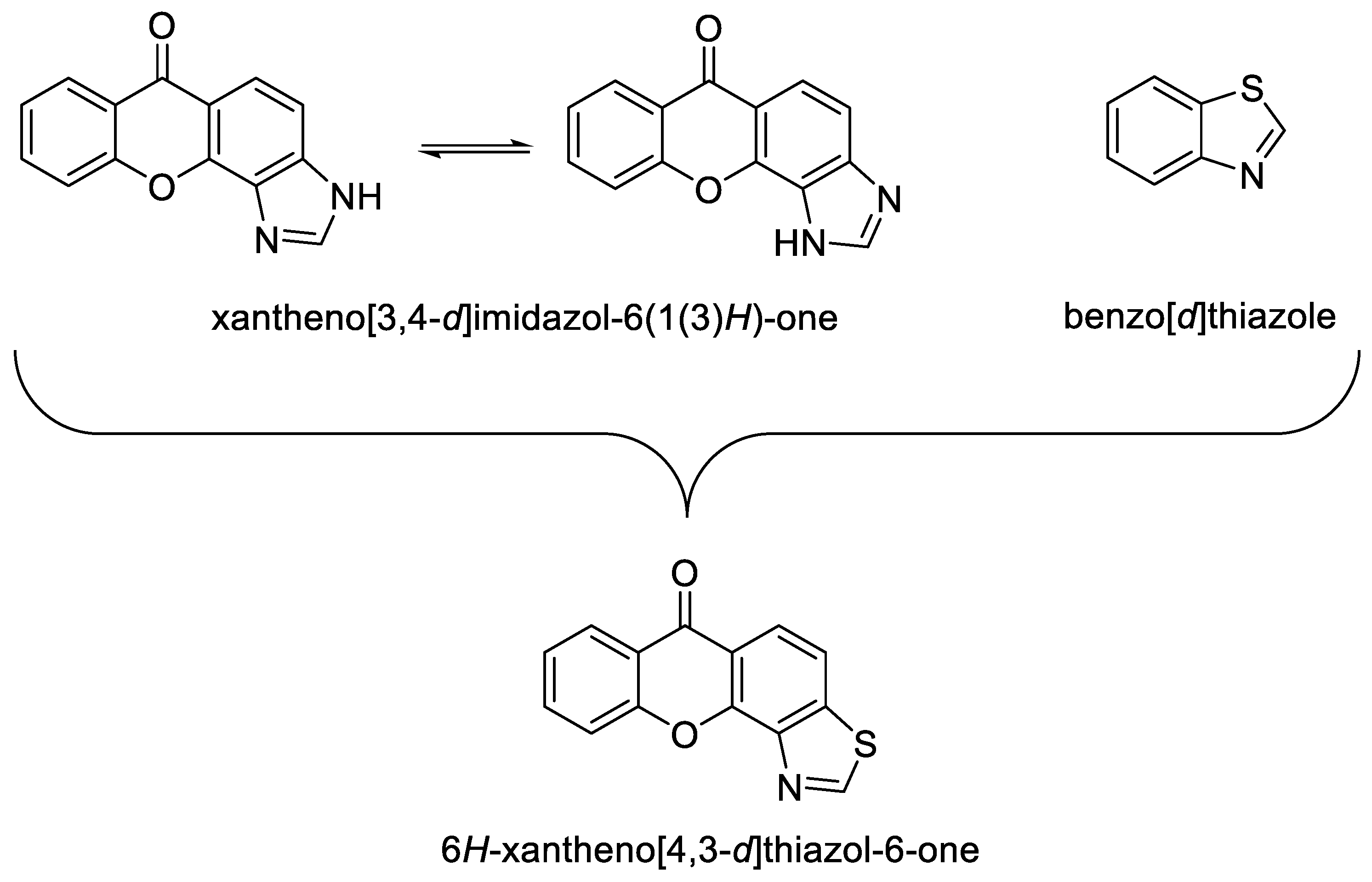
2. Results and Discussion
2.1. General
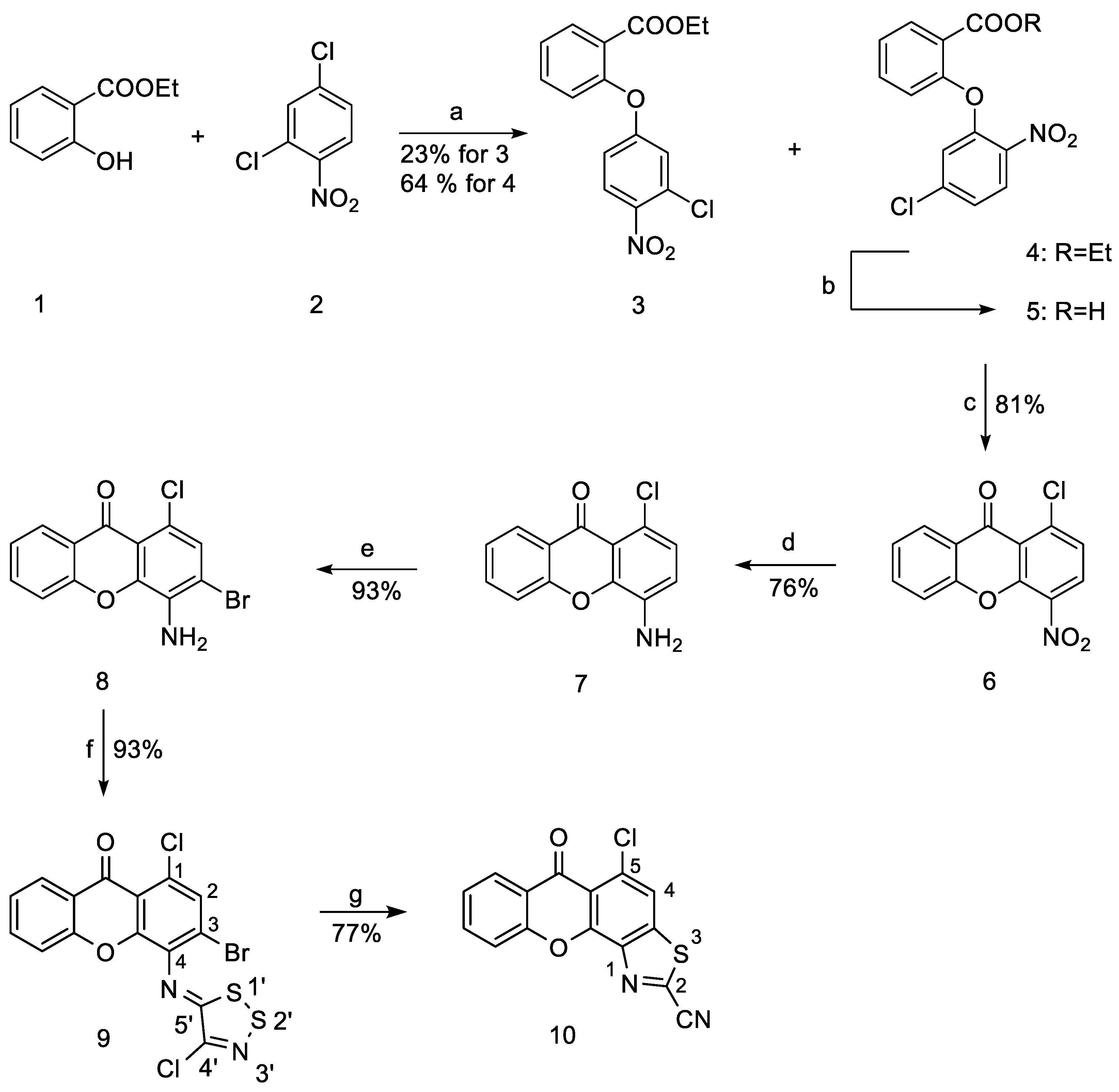
2.1.1. Synthesis of Ethyl 2-(5-Chloro-2-nitrophenoxy)benzoate (4)
2.1.2. Synthesis of 1-Chloro-4-nitro-9H-xanthen-9-one (6)
2.1.3. Synthesis of 4-Amino-1-chloro-9H-xanthen-9-one (7)
2.1.4. Synthesis of 4-Amino-3-bromo-1-chloro-9H-xanthen-9-one (8)
2.1.5. Synthesis of 3-Bromo-1-chloro-4-((4-chloro-5H-1,2,3-dithiazol-5-ylidene)amino)-9H-xanthen-9-one (9)
2.1.6. Synthesis of 5-Chloro-6-oxo-6H-xantheno[4,3-d]thiazole-2-carbonitrile (10)
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Malik, M.S.; Alsantali, R.I.; Jassas, R.S.; Alsimaree, A.A.; Syed, R.; Alsharif, M.A.; Kalpana, K.; Morad, M.; Althagafi, I.I.; Ahmed, S.A. Journey of Anthraquinones as Anticancer Agents—A Systematic Review of Recent Literature. RSC Adv. 2021, 11, 35806–35827. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, A.; Kalampaliki, A.D.; Gioti, K.; Hamdoun, S.; Giannopoulou, A.F.; Efferth, T.; Stravopodis, D.J.; Tenta, R.; Marakos, P.; Pouli, N.; et al. Synthesis of Novel Xanthone and Acridone Carboxamides with Potent Antiproliferative Activities. Arab. J. Chem. 2020, 13, 7953–7969. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, X. Current Scenario of Acridine Hybrids with Anticancer Potential. CTMC 2021, 21, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, I.K.; Magiatis, P.; Pouli, N.; Marakos, P.; Skaltsounis, A.-L.; Pratsinis, H.; Leonce, S.; Pierre, A. Design, synthesis, and antiproliferative activity of some new pyrazole-fused amino derivatives of the pyranoxanthenone, pyranothioxanthenone, and pyranoacridone ring systems: A new class of cytotoxic agents. J. Med. Chem. 2002, 45, 2599–2609. [Google Scholar] [CrossRef] [PubMed]
- Frasinyuk, M.; Chhabria, D.; Kartsev, V.; Dilip, H.; Sirakanyan, S.N.; Kirubakaran, S.; Petrou, A.; Geronikaki, A.; Spinelli, D. Benzothiazole and Chromone Derivatives as Potential ATR Kinase Inhibitors and Anticancer Agents. Molecules 2022, 27, 4637. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Sinhmar, A.; Sharma, A.; Rajak, H.; Pathak, D.P. Medicinal significance of benzothiazole scaffold: An insight view. Enzyme Inhib. Med. Chem. 2013, 28, 240–266. [Google Scholar] [CrossRef]
- Bhat, M.; Belagali, S.L. Structural Activity Relationship and Importance of Benzothiazole Derivatives in Medicinal Chemistry: A Comprehensive Review. Mini-Rev. Org. Chem. 2020, 17, 323–350. [Google Scholar] [CrossRef]
- Fruit, C.; Couly, F.; Bhansali, R.; Rammohan, M.; Lindberg, M.F.; Crispino, J.D.; Meijer, L.; Besson, T. Biological Characterization of 8-Cyclopropyl-2-(Pyridin-3-Yl)Thiazolo[5,4-f]Quinazolin-9(8H)-One, a Promising Inhibitor of DYRK1A. Pharmaceuticals 2019, 12, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrou, A.; Zagaliotis, P.; Theodoroula, N.F.; Mystridis, G.A.; Vizirianakis, I.S.; Walsh, T.J.; Geronikaki, A. Thiazole/Thiadiazole/Benzothiazole Based Thiazolidin-4-One Derivatives as Potential Inhibitors of Main Protease of SARS-CoV-2. Molecules 2022, 27, 2180. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V.; Kostakis, I.K.; Pouli, N.; Marakos, P.; Kousidou, O.C.; Tzanakakis, G.N.; Karamanos, N.K. Design, Synthesis, and Evaluation of the Antiproliferative Activity of a Series of Novel Fused Xanthenone Aminoderivatives in Human Breast Cancer Cells. J. Med. Chem. 2007, 50, 1716–1719. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, I.K.; Tenta, R.; Pouli, N.; Marakos, P.; Skaltsounis, A.-L.; Pratsinis, H.; Kletsas, D. Design, Synthesis, and Antiproliferative Activity of Some Novel Aminosubstituted Xanthenones, Able to Overcome Multidrug Resistance toward MES-SA/Dx5 Cells. Bioorg. Med. Chem. Lett. 2005, 15, 5057–5060. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.; Koutentis, P. The Reaction of 4,5-Dichloro-1,2,3-Dithiazolium Chloride with Sulfimides: A New Synthesis of N-Aryl-1,2,3-Dithiazolimines. Molecules 2009, 14, 2356–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letribot, B.; Delatouche, R.; Rouillard, H.; Bonnet, A.; Chérouvrier, J.-R.; Domon, L.; Besson, T.; Thiéry, V. Synthesis of 2-Mercapto-(2-Oxoindolin-3-Ylidene)Acetonitriles from 3-(4-Chloro-5H-1,2,3-Dithiazol-5-Ylidene)Indolin-2-ones. Molecules 2018, 23, 1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuadro, A.M.; Alvarez-Buila, J. 4,5-Dichloro-1,2,3-dithiazolium chloride (Appel’s Salt): Reactions with N-nucleophiles. Tetrahedron 1994, 50, 10037–10046. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraskevas, K.; Iliopoulos-Tsoutsouvas, C.; Georgiou, E.A.; Kostakis, I.K. 5-Chloro-6-oxo-6H-xantheno[4,3-d]thiazole-2-carbonitrile. Molbank 2022, 2022, M1489. https://doi.org/10.3390/M1489
Paraskevas K, Iliopoulos-Tsoutsouvas C, Georgiou EA, Kostakis IK. 5-Chloro-6-oxo-6H-xantheno[4,3-d]thiazole-2-carbonitrile. Molbank. 2022; 2022(4):M1489. https://doi.org/10.3390/M1489
Chicago/Turabian StyleParaskevas, Konstantinos, Christos Iliopoulos-Tsoutsouvas, Eleftheria A. Georgiou, and Ioannis K. Kostakis. 2022. "5-Chloro-6-oxo-6H-xantheno[4,3-d]thiazole-2-carbonitrile" Molbank 2022, no. 4: M1489. https://doi.org/10.3390/M1489
APA StyleParaskevas, K., Iliopoulos-Tsoutsouvas, C., Georgiou, E. A., & Kostakis, I. K. (2022). 5-Chloro-6-oxo-6H-xantheno[4,3-d]thiazole-2-carbonitrile. Molbank, 2022(4), M1489. https://doi.org/10.3390/M1489