Synthesis and Characterization of 3-Methyl-1-(4-(trifluoromethyl)phenyl)indeno [1,2-c]pyrazol-4(1H)-one
Abstract
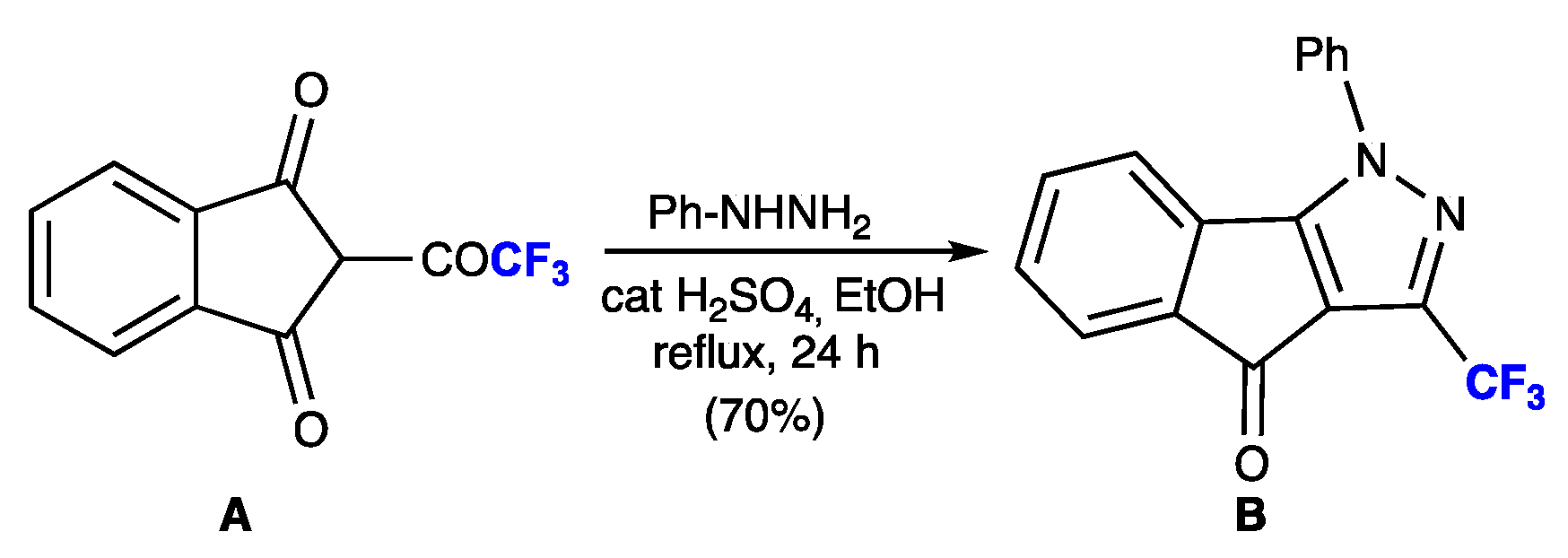
1. Introduction
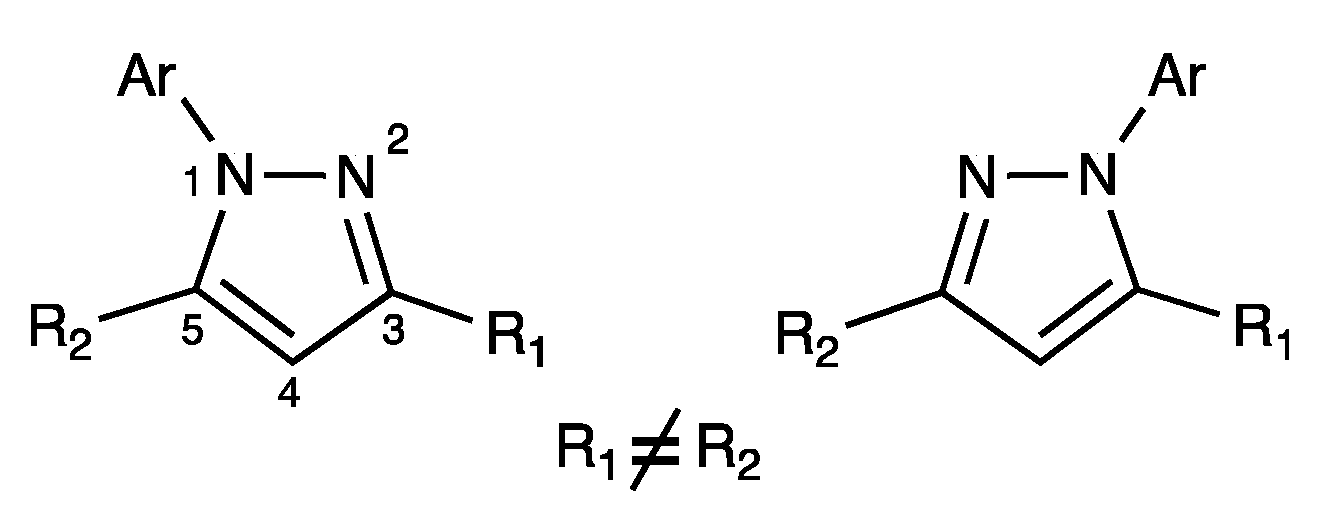
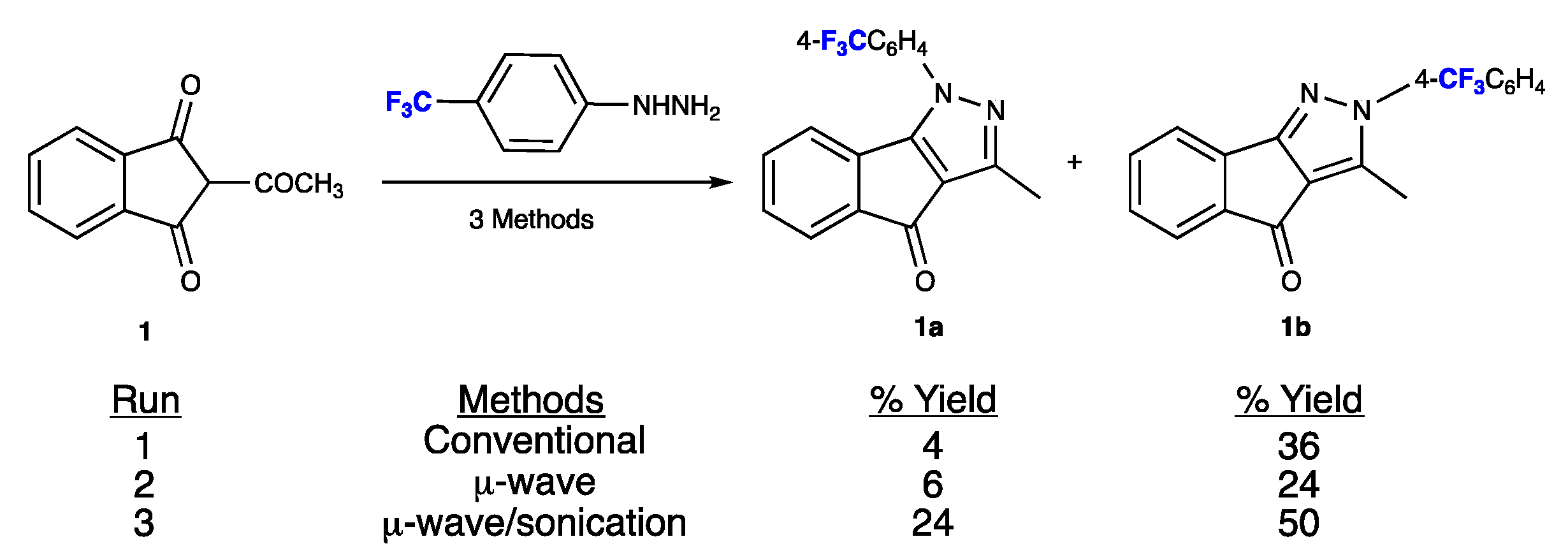
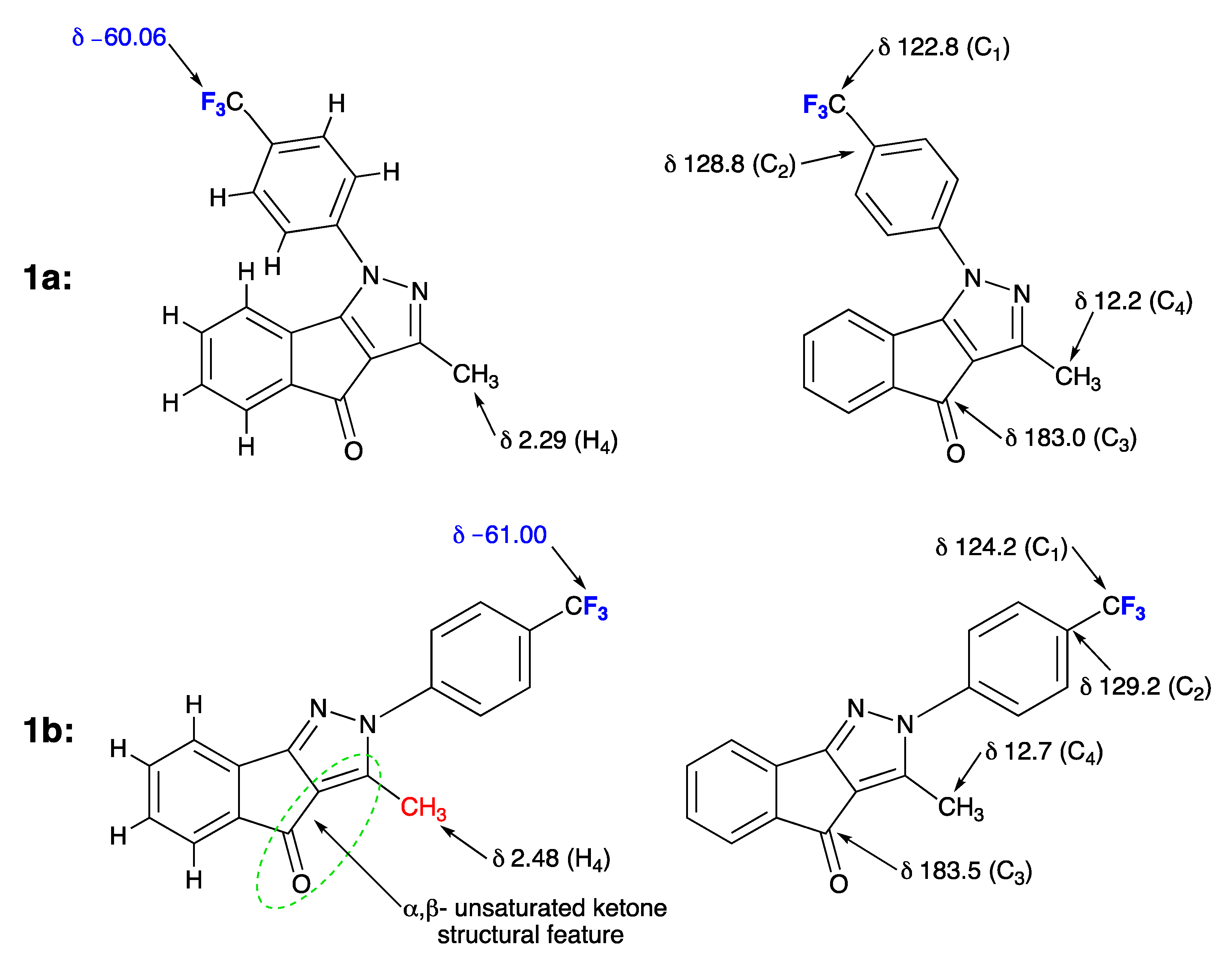
2. Results
3. Discussion
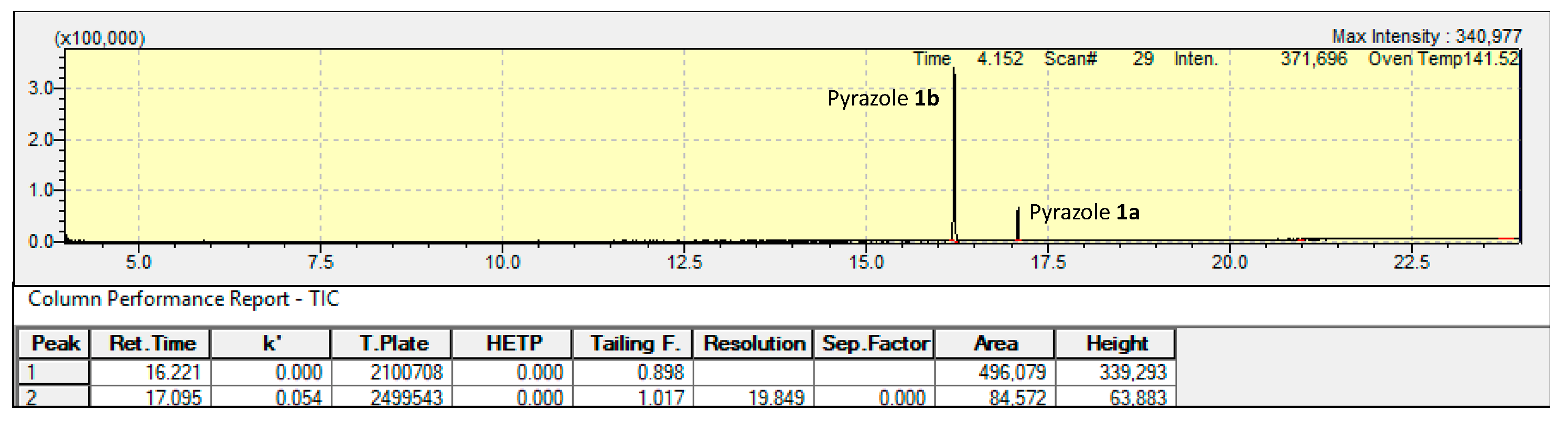
3.1. Synthesis and Purification
3.2. Spectral Characterization
4. Materials and Methods
4.1. Instrumentation
4.2. Chemicals
4.3. Methods for the Preparation and Isolation of 3-Methyl-1-(4-(trifluoromethyl)phenyl)indeno[1,2-c]pyrazol-4(1H)-one (1a)
4.3.1. Run 1
4.3.2. Run 2
4.3.3. Run 3
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef] [PubMed]
- Sloop, J.; Holder, C.; Henary, M. Selective Incorporation of Fluorine in Pyrazoles. Eur. J. Org. Chem. 2015, 16, 3405–3422. [Google Scholar] [CrossRef]
- Sloop, J.; Bumgardner, C.; Loehle, W.D. Synthesis of Fluorinated Heterocycles. J. Fluor. Chem. 2002, 118, 135–147. [Google Scholar] [CrossRef]
- Wu, M.; Wang, R.; Chen, F.; Chen, W.; Zhou, Z.; Yi, W. Synthesis of Indenopyrazole Frameworks via Cascade C–H Functionalization/[3 + 2] Dipolar Cycloaddition/Aromatization Rearrangement Reactions. Org. Lett. 2020, 22, 7152–7157. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Kumar, S. 5-Aminopyrazole as precursor in design and synthesis of fused pyrazoloazines. Beilstein J. Org. Chem. 2018, 14, 203–242. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, I.; Hawass, M.; Sanad, S.; Elwahy, A. Synthesis of various pyrazole-fused heterocyclic systems using pyrazole-4-carbaldehydes as versatile precursors. Arkivoc 2021, ix, 42–72. [Google Scholar] [CrossRef]
- Nugiel, D.; Vidwans, A.; Etzkorn, A.; Rossi, R.; Benfield, P.; Burton, C.; Cox, S.; Doleniak, D.; Seitz, S. Synthesis and Evaluation of Indenopyrazoles as Cyclin-Dependent Kinase Inhibitors. 2. Probing the Indeno Ring Substituent Pattern. J. Med. Chem. 2002, 45, 5224–5232. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, H.; Fukashiro, S.; Ban, H.S.; Nakamura, H. Discovery of Indenopyrazoles as a New Class of Hypoxia Inducible Factor (HIF)-1 Inhibitors. ACS Med. Chem. Lett. 2013, 4, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Mykhailiuk, P. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2021, 121, 1670–1715. [Google Scholar] [CrossRef] [PubMed]
- Gillis, E.; Eastman, K.; Hill, M.; Donnelly, D.; Meanwell, N. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.; Talley, J.; Bertenshaw, S.; Carter, J.; Collins, P.; Docter, S.; Graneto, M.; Lee, L.; Malecha, J.; Miyashiro, J.; et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: Identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1h-pyrazol-1-yl]benzenesulfonamide (sc-58635, celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- van Klink, J.W.; Larsen, L.; Perry, N.; Weavers, R.; Cook, G.; Bremer, P.; MacKenzie, A.; Kirikae, T. Triketones active against antibiotic-resistant bacteria: Synthesis, structure-activity relationships, and mode of action. Bioorganic Med. Chem. 2005, 13, 6651–6662. [Google Scholar] [CrossRef] [PubMed]
- Sloop, J.; Boyle, P.; Fountain, A.W.; Gomez, C.; Jackson, J.; Pearman, W.; Schmidt, R.; Weyand, J. Novel Fluorinated Indanone, Tetralone and Naphthone Derivatives: Synthesis and Unique Structural Features. Appl. Sci. 2012, 2, 61–99. [Google Scholar] [CrossRef]
- Sloop, J.; Jackson, J.; Schmidt, R. Microwave-Mediated Pyrazole Fluorinations Using Selectfluor®. Heteroat. Chem. 2009, 20, 341–345. [Google Scholar] [CrossRef]
- Sloop, J.; Lechner, B.; Washington, G.; Bumgardner, C.; Loehle, W.; Creasy, W. Pyrazole Formation: Examination of Kinetics and Mechanistic Pathways. Int. J. Chem. Kinet. 2008, 40, 370–383. [Google Scholar] [CrossRef]
- Boyle, P.; Breaud, D.; Churley, M.; Coppock, P., Jr.; Encarnacion-Thomas, E.; Fernandes, C.; Fountain, A.; Gomez, C.; Guzman, A.; Jackson, J.; et al. Synthesis and Structural Features of Indanone, Tetralone and Naphthone Derivatives: Selective Fluorination and Condensation Products, Chapter 2. In Current Perspectives on Chemical Sciences, Volume 2; Sutar, H., Ed.; Book Publisher International: London, UK, 2020; 16p. [Google Scholar] [CrossRef]
- Dolbier, W. Guide to Fluorine NMR For Organic Chemists; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
| Entry | Possible Intermediate & Pyrazole Structures | Rf (CH2Cl2) | Rf (EtOAc/Hexane) | Relative Spot Size/ Color (1 = Largest; 6 = Smallest) |
|---|---|---|---|---|
| 1/2 | 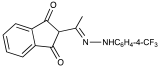 or | 0.94 | 0.97 | 2/Orange |
| 1/2 | 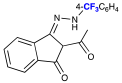 | 0.81 | 0.85 | 4/Yellow-orange |
| 3 | 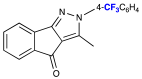 | 0.46 | 0.75 | 1/Pale brown |
| 4 | 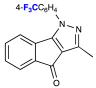 | 0.36 | 0.58 | 3/Pale brown |
| 5/6 |  or | 0.16 | 0.46 | 5/Pale brown |
| 5/6 | 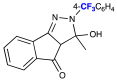 | 0.08 | 0.38 | 6/Pale brown |
| Reactants | ||||
| 7 |  | 0.06 | 0.15 | Not present |
| 8 | 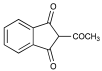 | 0.28 | 0.66 | Not present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, L.; Park, S.H.; Sloop, J. Synthesis and Characterization of 3-Methyl-1-(4-(trifluoromethyl)phenyl)indeno [1,2-c]pyrazol-4(1H)-one. Molbank 2022, 2022, M1483. https://doi.org/10.3390/M1483
Lam L, Park SH, Sloop J. Synthesis and Characterization of 3-Methyl-1-(4-(trifluoromethyl)phenyl)indeno [1,2-c]pyrazol-4(1H)-one. Molbank. 2022; 2022(4):M1483. https://doi.org/10.3390/M1483
Chicago/Turabian StyleLam, Linh, Sang H. Park, and Joseph Sloop. 2022. "Synthesis and Characterization of 3-Methyl-1-(4-(trifluoromethyl)phenyl)indeno [1,2-c]pyrazol-4(1H)-one" Molbank 2022, no. 4: M1483. https://doi.org/10.3390/M1483
APA StyleLam, L., Park, S. H., & Sloop, J. (2022). Synthesis and Characterization of 3-Methyl-1-(4-(trifluoromethyl)phenyl)indeno [1,2-c]pyrazol-4(1H)-one. Molbank, 2022(4), M1483. https://doi.org/10.3390/M1483








