One-Pot Synthesis of Dioxime Oxalates
Abstract
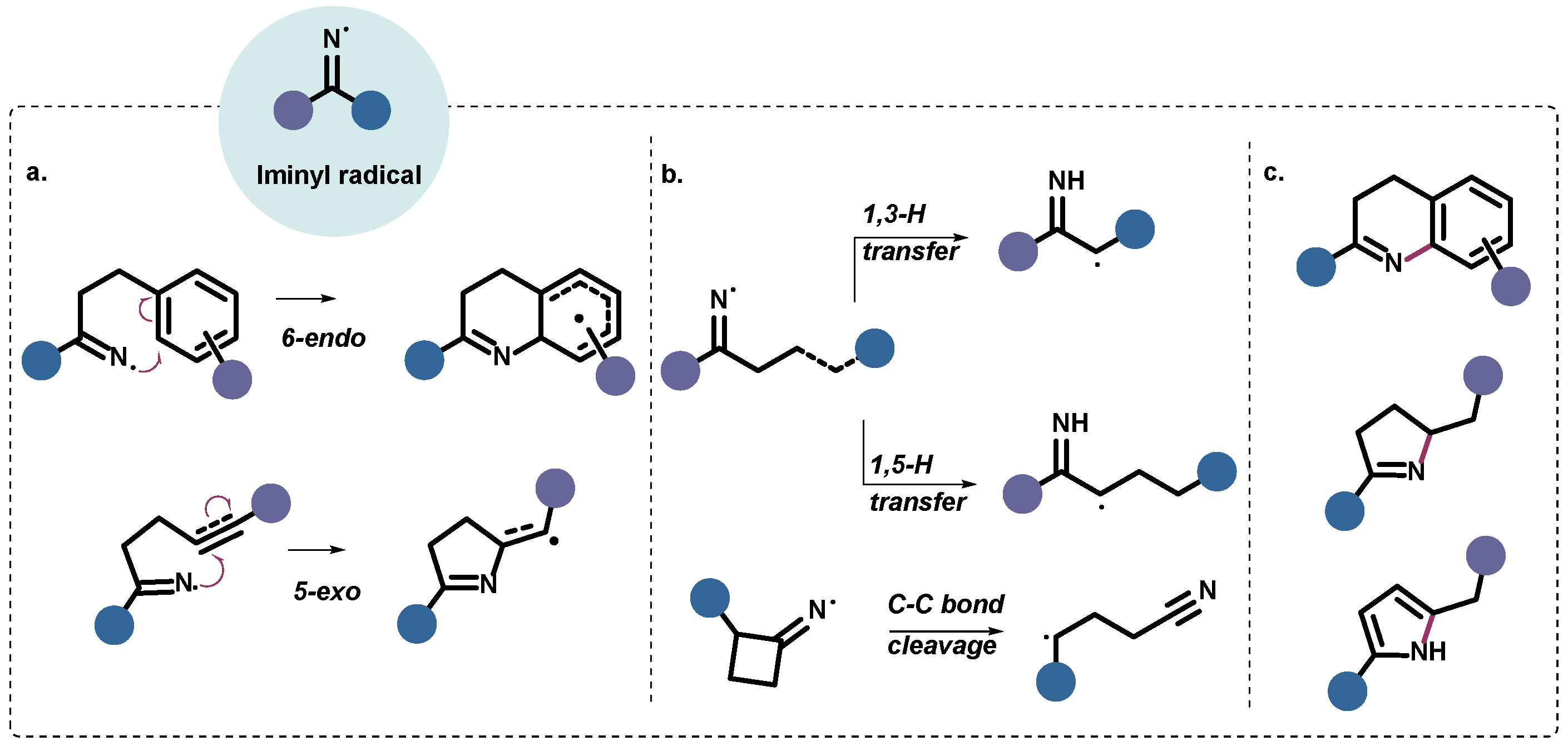
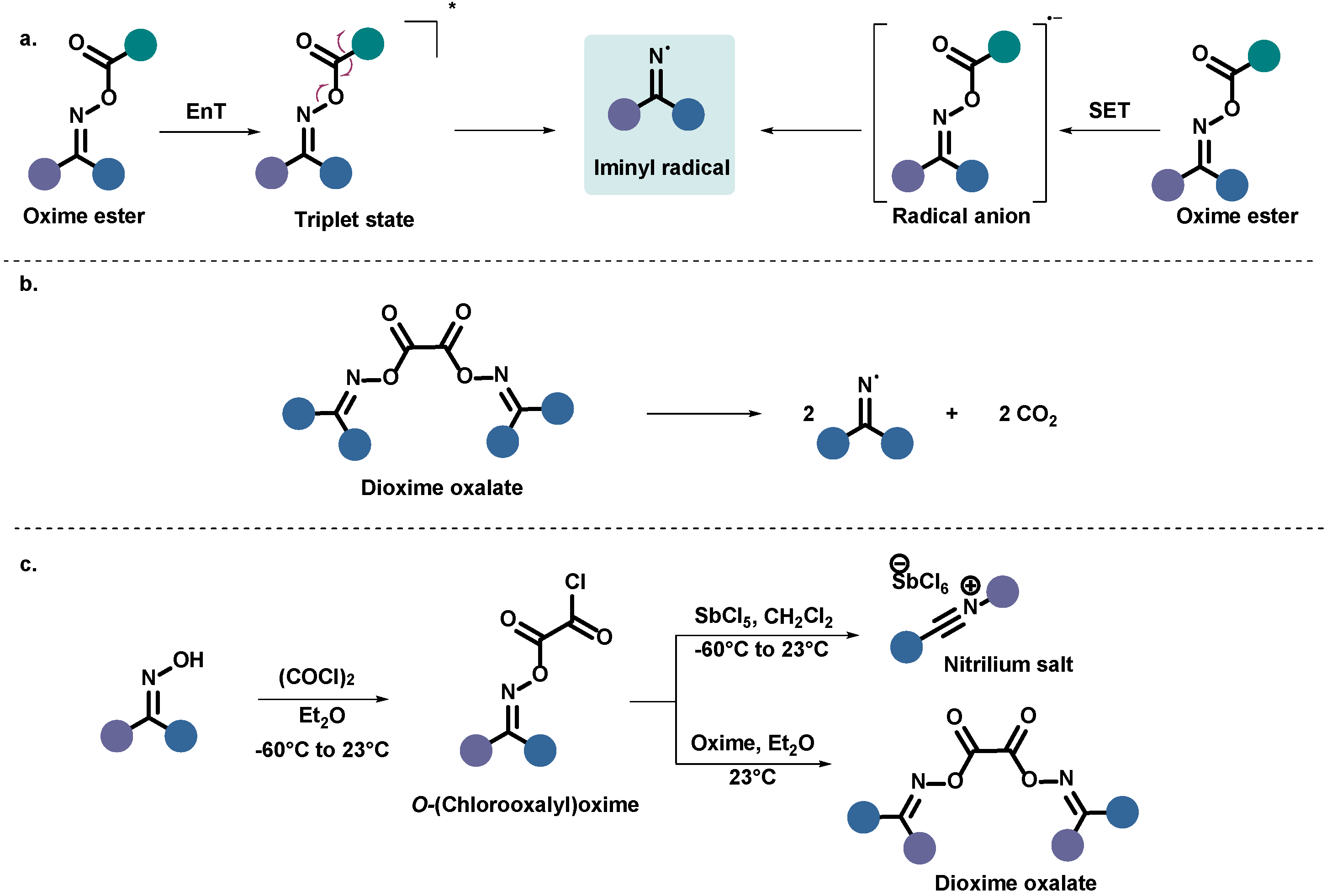
1. Introduction
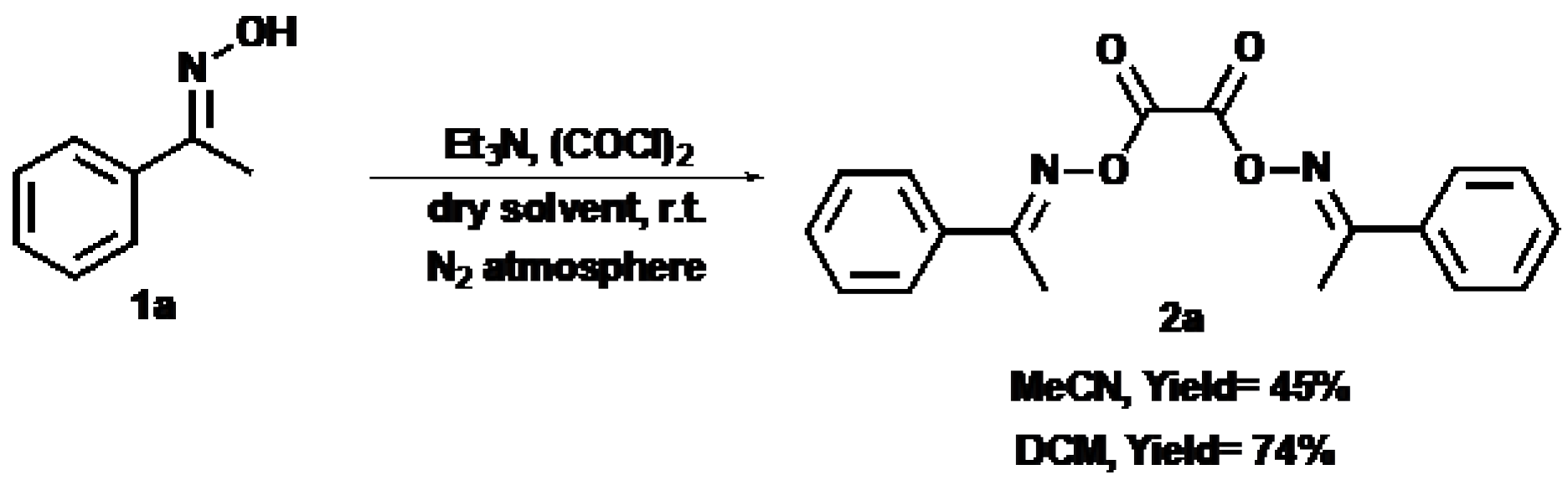
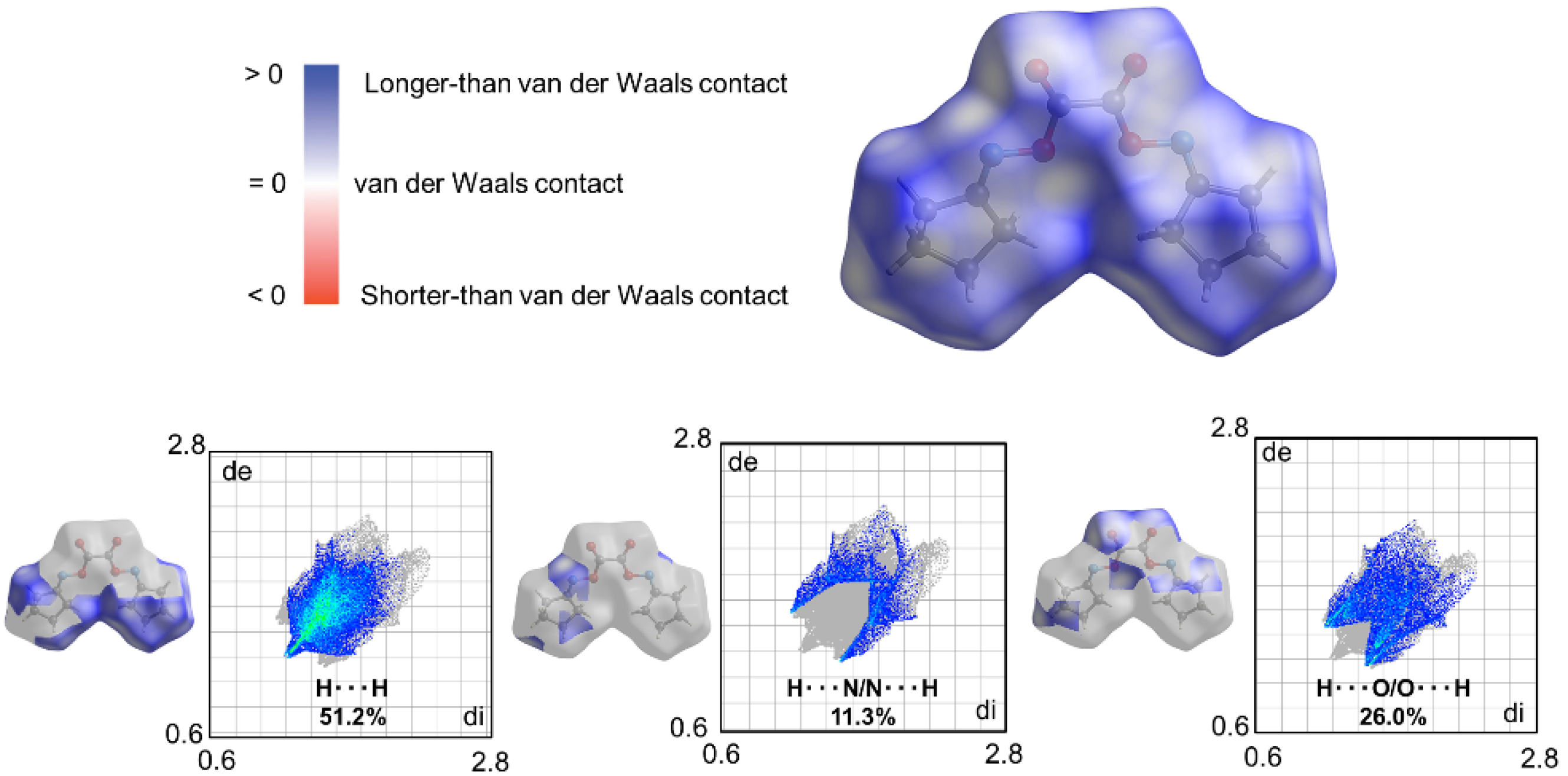
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Ketoximes
4.2.1. Acetophenone Oxime (1a)
4.2.2. Cyclopentanone Oxime (1b)
4.2.3. Cyclohexanone Oxime (1c)
4.2.4. 3-Phenylcyclobutan-1-one Oxime (1d)
4.2.5. 2,3-Dihydro-1H-Inden-1-one Oxime (1e)
4.3. Preparation of Dioxime Oxalates
4.3.1. O,O′-Oxalylbis(1-phenylacetophenone oxime) (2a)
4.3.2. O,O′-Oxalyldicyclopentanone Oxime (2b)
4.3.3. O,O′-Oxalyldicyclohexanone Oxime (2c)
4.3.4. O,O′-Oxalylbis(3-phenyl-3-phenylcyclobutan-1-one oxime) (2d)
4.3.5. O,O′-Oxalylbis(2,3-dihydro-1H-2,3-dihydro-1H-inden-1-one oxime) (2e)
4.4. Characterization Techniques
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Chemical Society National Historic Chemical Landmarks. Moses Gomberg and Organic Free Radicals. Available online: http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/freeradicals.html (accessed on 1 September 2022).
- Symons, M.C.R. The importance of imino radicals (R2C=N) as reaction intermediates. Tetrahedron 1973, 29, 615. [Google Scholar] [CrossRef]
- Roberts, B.P.; Winter, J.N. Electron spin resonance studies of radicals derived from organic azides. J. Chem. Soc. Perkin Trans. 1979, 2, 1353. [Google Scholar] [CrossRef]
- Griller, D.; Mendenhall, G.D.; Van Hoof, W.; Ingold, K.U. Kinetic applications of electron paramagnetic resonance spectroscopy. XV. Iminyl radicals. J. Am. Chem. Soc. 1974, 96, 6068. [Google Scholar] [CrossRef]
- Boivin, J.F.E.; Zard, S.Z. A New and Synthetically Useful Source of Iminyl Radicals. Tetrahedron Lett. 1991, 32, 4299–4302. [Google Scholar] [CrossRef]
- Boivin, J.F.E.; Zard, S.Z. Iminyl radicals: Part I. generation and intramolecular capture by an olefin. Tetrahedron 1994, 50, 1745–1756. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, H.; Anandb, D.; Zhou, L. Iminyl-Radical-Triggered C-C Bond Cleavage of Cycloketone Oxime Derivatives: Generation of Distal Cyano-Substituted Alkyl Radicals and Their Functionalization. Synthesis 2020, 52, 1585–1601. [Google Scholar] [CrossRef]
- Kitamura, M.; Narasaka, K. Synthesis of Aza-Heterocycles from Oximes by Amino-Heck Reaction. Chem. Rec. 2002, 2, 268–277. [Google Scholar] [CrossRef]
- Kitamura, M.; Narasaka, K. Amination with Oximes. Eur. J. Org. Chem. 2005, 2005, 4505–4519. [Google Scholar] [CrossRef]
- Zard, S.Z. Recent progress in the generation and use of nitrogen-centred radicals. Chem. Soc. Rev. 2008, 37, 1603–1618. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.C. The Oxime Portmanteau Motif: Released Heteroradicals Undergo Incisive EPR Interrogation and Deliver Diverse Heterocycles. Acc. Chem. Res. 2014, 47, 1406–1416. [Google Scholar] [CrossRef]
- Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016, 45, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- Jackman, M.M.; Cai, Y.; Castle, S.L. Recent Advances in Iminyl Radical Cyclizations. Synthesis 2017, 49, 1785–1795. [Google Scholar] [CrossRef]
- Li, J.; Zhang, P.; Jiang, M.; Yang, H.; Zhao, Y.; Fu, H. Visible light as a sole requirement for intramolecular C(sp3)–H imination. Org. Lett. 2017, 19, 1994–1997. [Google Scholar] [CrossRef] [PubMed]
- Strieth, F.S.; Glorius, F. Triplet Energy Transfer Photocatalysis: Unlocking the Next Level. Chem. 2020, 6, 1888–1903. [Google Scholar] [CrossRef]
- Rykaczewski, K.A.; Wearing, E.R.; Blackmun, D.E.; Schindler, C.S. Reactivity of oximes for diverse methodologies and synthetic applications. Nat. Synth. 2022, 1, 24–36. [Google Scholar] [CrossRef]
- Walton, J.C. Synthetic Strategies for 5- and 6-Membered Ring Azaheterocycles Facilitated by Iminyl Radicals. Molecules 2016, 21, 660. [Google Scholar] [CrossRef] [PubMed]
- Forrester, A.R.; Napier, R.J.; Thomson, R.H. Iminyls. Part 7. Intramolecular hydrogen abstraction; synthesis of heterocyclic analogues of α-tetralone. J. Chem. Soc. Perkin Trans. 1981, 1, 984–987. [Google Scholar] [CrossRef]
- Portela-Cubillo, F.; Scanlan, E.M.; Scott, J.S.; Walton, J.C. From dioxime oxalates to dihydropyrroles and phenanthridines viaiminyl radicals. Chem. Commun. 2008, 4189, 4189–4191. [Google Scholar] [CrossRef] [PubMed]
- Jochims, J.C.; Hehl, S.; Herzberger, S. Preparation and Beckmann Rearrangement of O-(Chlorooxalyl)oximes. Synthesis 1990, 1990, 1128–1133. [Google Scholar] [CrossRef]
- Chernykh, A.V.; Radchenko, D.S.; Chernykh, A.V.; Kondratov, I.S.; Tolmachova, N.A.; Datsenko, O.P.; Kurkunov, M.A.; Zozulya, S.X.; Kheylik, Y.P.; Bartels, K.; et al. Synthesis and Physicochemical Properties of 3-Fluorocyclobutylamines. Eur. J. Org. Chem. 2015, 2015, 6466–6471. [Google Scholar] [CrossRef]
- Ma, G.; Xu, Z.; Zhang, P.; Liu, J.; Hao, X.; Ouyang, J.; Liang, P.; You, S.; Jia, X. A Novel Synthesis of Rasagiline via a Chemoenzymatic Dynamic Kinetic Resolution. Org. Process Res. Dev. 2014, 18, 1169–1174. [Google Scholar] [CrossRef]
- Damljanović, I.; Vukićević, M.; Vukićević, D.R. A Simple Synthesis of Oximes. Monatsh. Chem. 2006, 137, 301–305. [Google Scholar] [CrossRef]
- Horáková, E.; Drabina, P.; Brůčková, L.; Štěpánková, Š.; Vorčáková, K.; Sedlák, M. Synthesis and in vitro evaluation of novel N-cycloalkylcarbamates as potential cholinesterase inhibitors. Monatsh. Chem. 2017, 148, 2143–2153. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]


| Entry | Oxime (1) | Dioxime Oxalate (2), Yield (%) 1 |
|---|---|---|
| 1 | 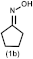 | (2b), 60 |
| 2 |  | (2c), 60 2 |
| 3 |  | (2d), 89 |
| 4 |  | (2e), 94 |
| Crystal Data | |
|---|---|
| Chemical Formula | C12H16N2O4 |
| Mr | 252.27 |
| Crystalline system, space group | Monoclinic, I2/c |
| a, b, c (Å) | 8.9695 (11), 11.2015 (11), 13.3635 (15) |
| α, β, γ (°) | 90, 109.087 (13), 90 |
| Volume, (Å3) | 1268.8 (3) |
| ρ, kg m−3 | 1.321 |
| Z | 4 |
| Temperature, (K) | 298 (2) |
| Radiation type | Cu Kα |
| μ (mm−1) | 0.84 |
| Theta range for data collection | 5.278° < 2θ < 76.337° |
| Index range | −11 ≤ h ≤ 11, |
| −13 ≤ k ≤ 14, | |
| −15 ≤ l ≤ 16 | |
| Data collection | |
| Diffractometer | SuperNova, Dual, Cu at zero, Atlas |
| Absorption correction | Multi-scan method CrysAlis PRO 1.171.41.119a (Rigaku Oxford Diffraction, 2021) |
| Tmin, Tmax | 0.908, 1.000 |
| No. of measured, independent, and observed reflections [I > 2σ(I)] | 5200, 1314, 1156 |
| Rint | 0.034 |
| (sin θ/λ)max (Å−1) | 0.630 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.048, 0.142, 1.09 |
| No. of reflections | 1314 |
| Refined parameters | 83 |
| H-atoms treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.23, −0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adarve-Cardona, L.; Ezenarro-Salcedo, D.; Macías, M.A.; Gamba-Sánchez, D. One-Pot Synthesis of Dioxime Oxalates. Molbank 2022, 2022, M1473. https://doi.org/10.3390/M1473
Adarve-Cardona L, Ezenarro-Salcedo D, Macías MA, Gamba-Sánchez D. One-Pot Synthesis of Dioxime Oxalates. Molbank. 2022; 2022(4):M1473. https://doi.org/10.3390/M1473
Chicago/Turabian StyleAdarve-Cardona, Laura, David Ezenarro-Salcedo, Mario A. Macías, and Diego Gamba-Sánchez. 2022. "One-Pot Synthesis of Dioxime Oxalates" Molbank 2022, no. 4: M1473. https://doi.org/10.3390/M1473
APA StyleAdarve-Cardona, L., Ezenarro-Salcedo, D., Macías, M. A., & Gamba-Sánchez, D. (2022). One-Pot Synthesis of Dioxime Oxalates. Molbank, 2022(4), M1473. https://doi.org/10.3390/M1473








