Abstract
Two new indolo-glycyrrhetic acid derivatives containing cyano-substitutent at C30 have been synthesized, and their anti-influenza activity has been evaluated in vitro. The resulting data demonstrated moderate inhibitory activity against the H1N1 virus with IC50 29 and 23 μM, respectively. The value of SI 18 and 21 confirm low toxicity and potential of new compounds for following research and development of new biological agents.
1. Introduction
As the pathogen that caused the first influenza virus pandemic in this century, the influenza A (H1N1) virus has caused continuous harm to human public health. At present, amantadine, rimantadine, oseltamivir and zanamivir are the main antiviral drugs used to suppress influenza viruses. However, it has been reported that the influenza A virus’s drug resistance to oseltamivir is gradually increasing [].
Hence, the development of new antiviral compounds is desperately needed, so a lot of articles have been dedicated to the synthesis of this type of drug [,,]. Among them, much attention has been paid to medicinal plants, which exhibit antiviral activities and therapeutic effects. Extensive research has demonstrated that natural oleanane-type pentacyclic triterpenes, for example, 3β-hydroxy-11-oxo-olean-12(13)-en-30-oic acid (GA), possess several beneficial pharmacological activities including anti-viral, anti-cancer, anti-inflammatory, anti-microbial, antifungal and hepatoprotective properties []. Moreover, different methods of modification of GA have been widely used to synthesize many derivatives for many pharmacological applications. [,].
Thus, the oxidized analog of GA (3-oxo-GA) has been shown to possess the most potent anti-HCV activity with an IC50 value of 0.79 μM, which is nearly 200 times more than that of GA (IC50 159.4 μM) [].
GA derivatives bearing substituted indoles or N-phenylpyrazoles fused to their A-ring showed a 50% inhibitory concentration of PTP1B (protein-tyrosine phosphatase 1B enzyme) in a range of 2.5 to 10.1 µM. For example, the GA derivative containing a trifluoromethyl group in the 5′ carbon atom of the indole cycle exhibited non-competitive inhibition of PTP1B as well as higher potency (IC50 = 2.5 µM) []. An analog of GA with ortho-methoxybenzyl pyrozole in the A-ring showed a strong anti-inflammatory effect and high affinity for HMGB1 with a Kd value of 12.5 μM [] (Figure 1).
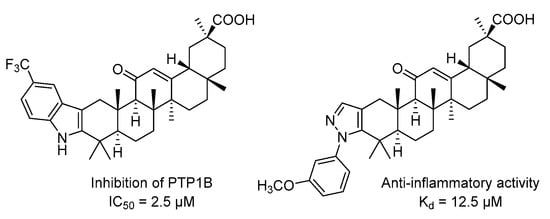
Figure 1.
Heterocyclic derivatives of GA with biological activity [,].
A series of amide derivatives of GA were investigated for their antiviral activity [,]. Thus, a compound containing a propanol-2 fragment at C30 was active (EC50 4.95 µM, SI 38.38) against TK+ and TK-strains of Herpes simplex virus type 1 (HSV-1) and sensitive and resistant to acyclovir (ACV) treatment [], whereas an amide containing an aminopyridine moiety had a strong inhibitory activity on ZIKV-induced CPE and viral protein translation in infected cells (IC50 0.13 μM, TI > 384) [].
On the other hand, cyano-fragment also has a positive influence on the biological profile of the molecule. Thus, methyl soloxolone (analogous to CDDO-OMe) contains a cyano group at the C2 position and has high antitumor activity, including against multidrug-resistant cell lines [].
In the present study, two new indolo-GA derivatives with cyano-substitution at C30 were synthesized and their cytotoxicity and anti-influenza properties were evaluated.
2. Results and Discussion
2.1. Chemistry
The compounds were synthesized, as outlined in Scheme 1. At first, 2,3-indolo-derivative 1 was prepared by oxidation of starting 3-hydroxo-glycyrrhetinic acid followed by treatment with PhNHNH2 (yield 89%) []. Then compound 1 was treated with chloroacetonitrile or 4-bromobutyronitrile to afford 2 and 3 (yield 90% and 92%, respectively). The structure of the newly synthesized compounds was confirmed by 1H- and 13C-NMR spectroscopy data. The signal of the carbon atom of the amide bond was observed at δ 176 ppm (13C), the CN-group at δ 114 (for 2) and 118 (for 3) ppm. (See Supplementary Material).

Scheme 1.
Synthesis of cyanoesters of 2,3-indolo-glycyrrhetinic acid. Reagent and conditions: i. PhNHNH2, AcOH, reflux, 2 h; ii. ClCH2CN (for 3) or BrCH2CH2CH2CN (for 4), K2CO3, DMF, 60 °C, 2 h.
2.2. Biological Assay
Cytotoxicity and anti-influenza properties of the derivatives 2 and 3 were studied in MDCK cell culture against influenza virus A/PuertoRico/8/34 (H1N1). Compounds at a range of concentrations (3.7–300 µg/mL) were tested followed by the calculation of their CC50′s and IC50′s, converting these values from µg/mL to micromoles and the calculation of selectivity indices. Oseltamivir carboxylate was used as a reference compound. As can be seen in Table 1, the study of cytotoxic activity demonstrated a complete absence of cytotoxicity activity even at the highest concentration used (300 µg/mL). At the same time, cyano-ester derivatives 2 and 3 exhibited moderate inhibitory activity against the H1N1 virus with IC50 29 and 23 μM, respectively. The values of SI 18 and 21 were moderate, indicating low toxicity and the potential for new compounds with further modifications to obtain more active and non-toxic compounds.

Table 1.
Cytotoxicity and anti-influenza data of the derivatives 2 and 3.
Derivatives of triterpenes were previously shown to be effective in the inhibition of influenza virus reproduction. Indeed, they were demonstrated to bind to viral hemagglutinin, thus disrupting the interaction of HA with the cellular receptor and further penetration of viruses into host cells. In an in vivo model, in addition to direct virus-inhibiting activity, these compounds exert anti-inflammatory effects as well as several indirect effects, including reduced activation of NF-kB, JNK and p38, redox-sensitive signaling events that are known to be relevant for influenza A virus replication []. Further studies, therefore, should decipher the exact targets and mechanisms of the anti-viral activity of lead compounds identified in our work and determine the range of their virus-inhibiting effects.
The nature of triterpene scaffold modification can play two roles. First, these modifications can be considered as having an indirect effect, i.e., facilitation of membrane penetration or prolongation of the half-life of a compound. Otherwise, they may exert direct and specific inhibiting functions in binding to viral and/or cellular targets. Further studies should be devoted to these issues.
3. Materials and Methods
The spectra were recorded at the Center for the Collective Use ‘Chemistry’ of the Ufa Institute of Chemistry of the UFRC RAS and RCCU “Agidel” of the UFRC RAS. 1H- and 13C-NMR spectra were recorded on a “Bruker Avance-III” (Bruker, Billerica, MA, USA, 500 and 125.5 MHz, respectively, δ, ppm, Hz) in CDCl3, internal standard—tetramethylsilane. Mass spectra were obtained on a liquid high-resolution chromatograph–mass spectrometer Agilent LC/Q-TOF 6530 (Santa Clara, CA, USA). Melting points were detected on a microtable «Rapido PHMK05» (Nagema, Dresden, Germany). Optical rotations were measured on a polarimeter Perkin–Elmer 241 MC (PerkinElmer, Waltham, MA, USA) in a tube length of 1 dm. Elemental analysis was performed on a Euro EA-3000 CHNS analyzer (Eurovector, Milan, Italy), the main standard is acetanilide. Thin-layer chromatography analyzes were performed on Sorbfil plates (Sorbpolimer, Krasnodar, Russia), using the solvent system chloroform–ethyl acetate, 40:1. Substances were detected by a 10% solution of sulfuric acid solution with subsequent heating at 100–120 °C for 2–3 min. All chemicals were of reagent grade (Sigma-Aldrich). 2,3-Indolo- glycyrrhetic acid 1 was synthesized by Fisher reactions as described in [].
3.1. General Method for Synthesis of Compounds 2 and 3
The solution of 1 (500 mg, 1 mmol), K2CO3 (135 mg, 0.98 mmol), and chloroacetonitrile (0.08 mL, 1.3 mmol) or 4-bromobutyronitrile (0.13 mL, 1.3 mmol) in DMF (20 mL) was stirred at 60 °C for 2 h. After cooling to room temperature, the mixture was poured into ice-cold water and filtered. The residue was filtered off, washed with water and dried, then purified by column chromatography on SiO2 using a mixture of petroleum ether—EtOAc (10:1) as eluent.
3.1.1. Cyanomethyl Ester 2,3-Indolo-olean-11-oxo-12(13)-en-30-oat (2)
90%, yellow powder. mp: 227–229 °C, [α]20 D +7 (c 0.75, CH2Cl2). 1H-NMR (δ, ppm, CDCl3, 500 MHz): 7.89 (1H, br. s, NH), 7.50–7.03 (4H, m, Harom), 5.74 (1H, s, H-12), 4.24–3.91 (2H, m, OCH2), 2.69–1.44 (19H, m, CH, CH2), 1.42, 1.32, 1.25, 1.22, 1.19, 0.85 (21H, s, 7CH3). 13C-NMR (δ, ppm, CDCl3, 125.5 MHz): 199.8 (C-11), 174.9 (C-30), 168.5 (C-13), 140.1 (C-3), 136.2 (C-arom), 128.9 (C-arom), 128.3 (C-12), 120.9 (C-arom), 118.8 (C-arom), 118.5 (C-arom), 114.3 (CN), 110.3 (C-arom), 107.0 (C-2), 60.5, 53.0, 48.3, 48.2, 45.3, 44.2, 43.3, 40.9, 38.0, 37.7, 37.6, 34.0, 32.0, 31.8, 31.0, 28.5, 27.9., 26.5, 26.4, 23.5, 23.3, 18.5, 18.3, 16.0. Anal. Calcd for C38H48N2O3: C, 78.58; H, 8.33; N, 4.82. Found: C, 78.66; H, 8.39; N, 4.77. HRMS (ESI-MS) m/z: [M + H]+ 581.3752, calcd for C38H48N2O3: 580.8130.
3.1.2. 3-Cyanopropyl Ester Ester 2,3-Indolo-olean-11-oxo-12(13)-en-30-oat (3)
92%, yellow powder. mp: 219–221 °C, [α]20 D +12 (c 0.75, CH2Cl2). 1H-NMR (δ, ppm, CDCl3, 500 MHz): 7.78 (1H, br. s, NH), 7.41–6.93 (4H, m, Harom), 5.66 (1H, s, H-12), 4.61–3.84 (2H, m, OCH2), 2.56 (2H, m, OCH2CH2), 2.19–1.32 (21H, m, CH, CH2), 1.31, 1.21, 1.15, 1.11, 1.18, 0.75 (21H, s, 7CH3). 13C-NMR (δ, ppm, CDCl3, 125.5 MHz): 200.0 (C-11), 176.2 (C-30), 169.2 (C-13), 140.2 (C-3), 136.2 (C-arom), 128.9 (C-arom), 128.2 (C-12), 121.0 (C-arom), 118.9 (CN), 118.7 (C-arom), 118.5 (C-arom), 110.3 (C-arom), 107.1 (C-2), 62.3 (OCH2), 60.6, 53.0, 48.5, 45.4, 44.1, 43.5, 43.4, 41.1, 38.1, 37.8, 37.6, 34.1, 32.1, 32.0, 31.1, 28.7, 28.4, 26.6, 26.4, 24.8, 23.5, 23.4, 18.6, 18.4, 16.1, 14.4. Anal. Calcd for C40H52N2O3: C, 78.91; H, 8.61; N, 4.60. Found: C, 78.85; H, 8.74; N, 4.72. HRMS (ESI-MS) m/z: [M + H]+ 609.4057, calcd for C40H52N2O3: 608.8670.
4. Conclusions
Two new glycyrrhetinic acid derivatives with A-fused ring nitrogen-heterocycle- and cyano-substitutes at C30 were synthesized using the consequent modifications (oxidation, Fisher reaction, reaction with chloroacetonitrile or 4-bromobutyronitrile). The in vitro anti-influenza activity showed low toxicity (SI 18 and 21) and moderate inhibitory activity against the H1N1 virus with an IC50 29 and 23 μM, respectively. The obtained compounds are promising for the synthesis of tetrazolyl derivatives with potential strong biological activity.
Supplementary Materials
The following supporting information can be downloaded. Containing 1H- and 13C-NMR data of compounds 2 and 3; Biological assay.
Author Contributions
A.V.P. brought the idea, conducted the experiment, and did structure elucidation and prepared the manuscript; S.V.B. and V.V.Z. made biological experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Federal Program No 1021062311390-1-1.4.1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the all compounds are available from the authors.
References
- Lackenby, A.; Hungnes, O.; Dudman, S.G.; Meijer, A.; Paget, W.J.; Hay, A.J.; Zambon, M.C. Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill. 2008, 13, 8026. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, T.; Massari, S. Protein-protein interactions by influenza polymerase subunits as drug targets. Future Med. Chem. 2022, 14, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Pismataro, M.C.; Felicetti, T.; Bertagnin, C.; Nizi, M.G.; Bonomini, A.; Barreca, M.L.; Cecchetti, V.; Jochmans, D.; De Jonghe, S.; Neyts, J.; et al. 1,2,4-Triazolo[1,5-a]pyrimidines: Efficient one-step synthesis and functionalization as influenza polymerase PA-PB1 interaction disruptors. Eur. J. Med. Chem. 2021, 221, 113494. [Google Scholar] [CrossRef] [PubMed]
- Massari, S.; Nannetti, G.; Goracci, L.; Sancineto, L.; Muratore, G.; Sabatini, S.; Manfroni, G.; Mercorelli, B.; Cecchetti, V.; Facchini, M.; et al. Structural investigation of cycloheptathiophene-3-carboxamide derivatives targeting influenza virus polymerase assembly. J. Med. Chem. 2013, 56, 10118–10131. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Green, I.R.; Shamraiz, U.; Saleem, M.; Badshah, A.; Abbas, G.; Rehman, N.U.; Irshad, M. Therapeutic potential of glycyrrhetinic acids: A patent review (2010–2017). Expert Opin. Ther. Pat. 2018, 28, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.A.H.; Peng, Y.; Wang, Z.; Qiang, X.; Zhao, Q. Synthesis, antiviral, and antibacterial activity of the glycyrrhizic acid and glycyrrhetinic acid derivatives. Russ. J. Bioorg. Chem. 2022, 48, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, R.; Fan, J.; Guo, R. A Mini-review on structure-activity relationships of glycyrrhetinic acid derivatives with diverse bioactivities. Mini Rev. Med. Chem. 2022, 22, 2024–2066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Wang, P.R.; Chen, F.; Qian, X.J.; Jia, L.; Liu, X.J.; Li, L.; Jin, Y.S. Synthesis and anti-HCV activities of 18β-glycyrrhetinic acid derivatives and their in-silico ADMET analysis. Curr. Comput. Aided Drug Des. 2021, 17, 831–837. [Google Scholar] [CrossRef] [PubMed]
- De-la-Cruz-Martínez, L.; Duran-Becerra, C.; González-Andrade, M.; Páez-Franco, J.C.; Germán-Acacio, J.M.; Espinosa-Chávez, J.; Torres-Valencia, J.M.; Pérez-Villanueva, J.; Palacios-Espinosa, J.F.; Soria-Arteche, O.; et al. Indole- and pyrazole-glycyrrhetinic acid derivatives as PTP1B inhibitors: Synthesis, in vitro and in silico studies. Molecules 2021, 26, 4375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Z.; Yuan, H.; Chen, H.; Xie, N.; Wang, Z.; Sun, Q.; Zhang, W. Structure-based design of glycyrrhetinic acid derivatives as potent anti-sepsis agents targeting high-mobility group box-1. Bioorg. Chem. 2021, 106, 104461. [Google Scholar] [CrossRef] [PubMed]
- Zígolo, M.A.; Salinas, M.; Alché, L.; Baldessari, A.; Liñares, G.G. Chemoenzymatic synthesis of new derivatives of glycyrrhetinic acid with antiviral activity. Molecular docking study. Bioorg. Chem. 2018, 78, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Baltina, L.A.; Lai, H.C.; Liu, Y.C.; Huang, S.H.; Hour, M.J.; Baltina, L.A.; Nugumanov, T.R.; Borisevich, S.S.; Khalilov, L.M.; Petrova, S.F.; et al. Glycyrrhetinic acid derivatives as Zika virus inhibitors: Synthesis and antiviral activity in vitro. Bioorg. Med. Chem. 2021, 41, 116204. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.V.; Sen’kova, A.V.; Warszycki, D.; Salomatina, O.V.; Salakhutdinov, N.F.; Zenkova, M.A.; Logashenko, E.B. Soloxolone methyl inhibits influenza virus replication and reduces virus-induced lung inflammation. Sci. Rep. 2017, 7, 13968. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Geiler, J.; Naczk, P.; Sithisarn, P.; Leutz, A.; Doerr, H.W. Glycyrrhizin Exerts Antioxidative Effects in H5N1 Influenza A Virus-Infected Cells and Inhibits Virus Replication and Pro-Inflammatory Gene Expression. PLoS ONE 2011, 6, e19705. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).