1-Phenyl-3,3-di(1H-pyrazol-1-yl)propan-1-one
Abstract
1. Introduction
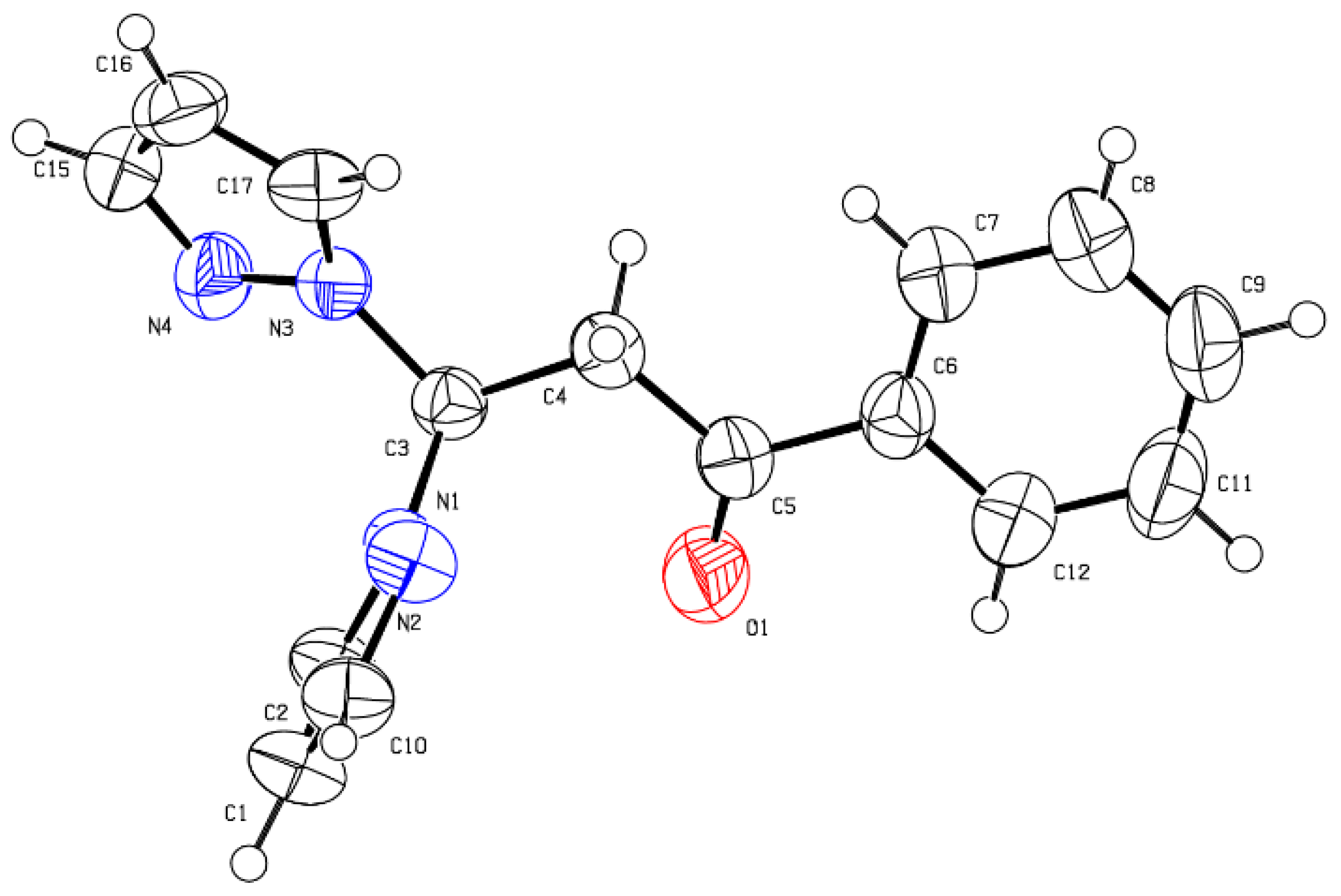
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavlov, P.T.; Goleneva, A.F.; Lesnov, A.E.; Prokhorova, T.S. Biological activity of some pyrazolone derivatives. Pharm. Chem. J. 1998, 32, 370. [Google Scholar] [CrossRef]
- Lupsor, S.; Aonofriesei, F.; Iovu, M. Antibacterial activity of aminals and hemiaminals of pyrazole and imidazole. MCRE 2011, 21, 3035. [Google Scholar] [CrossRef]
- Pal, A.; Ghosh, R.; Adarsh, N.; Sarkar, A. Pyrazole-tethered phosphine ligands for Pd(0): Useful catalysts for Stille, Kumada and Hiyama cross-coupling reactions. Tetrahedron 2010, 66, 5451–5458. [Google Scholar] [CrossRef]
- Huertas-Sanchez, A.; Luna-Giles, F.; Vinuelas-Zahinos, E.; Barros-Garcia, F.; Bernalte-García, A. Copper(II) halide coordination complexes with 2-(3,5-diphenyl-1-pyrazolyl)-2-thiazoline (DPhPyTn): Synthesis, characterization and crystal structures. Polyhedron 2015, 102, 394–400. [Google Scholar] [CrossRef]
- Fonseca, D.; Páez, C.; Ibarra, L.; García-Huertas, P.; Macías, M.A.; Triana-Chávez, O.; Hurtado, J.J. Metal complex derivatives of bis(pyrazol-1-yl)methane ligands: Synthesis, characterization and anti-Trypanosoma cruzi activity. Transit. Met. Chem. 2018, 44, 135. [Google Scholar] [CrossRef]
- Porchia, M.; Dolmella, A.; Gandin, V.; Marzano, C.; Pellei, M.; Peruzzo, V.; Refosco, F.; Santini, C.; Tisato, F. Neutral and charged phosphine/scorpionate copper(I) complexes: Effects of ligand assembly on their antiproliferative activity. Eur. J. Med. Chem. 2013, 59, 218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Fang, H.-J.; Hsu, S.C.N.; Jheng, N.-Y.; Chang, H.-C.; Ou, S.-W.; Peng, W.-T.; Lai, Y.-C.; Chen, J.-Y.; Chen, P.-L.; et al. Genome-wide association study of bipolar I disorder in the Han Chinese population. Polym. Bull. 2012, 70, 993. [Google Scholar] [CrossRef]
- Alkorta, I.; Claramunt, R.M.; Díez-Barra, E.; Elguero, J.; de la López, A.; López, C. The organic chemistry of poly(1H-pyrazol-1-yl)methanes. Coord. Chem. Rev. 2017, 339, 153. [Google Scholar] [CrossRef]
- Díez-Barra, E.; Guerra, J.; Hornillos, V.N.; Merino, S.; Tejeda, J. Double Michael addition of azoles to methyl propiolate: A straightforward entry to ligands with two heterocyclic rings. Tetrahedron Lett. 2004, 45, 6937. [Google Scholar] [CrossRef]
- Tsuchimoto, T.; Aoki, K.; Wagatsuma, T.; Suzuki, Y. Lewis acid catalyzed addition of pyrazoles to alkynes: Selective synthesis of double and single addition products. Eur. JOC 2008, 2008, 4035. [Google Scholar]
- Bhanuchandra, M.; Kuram, M.R.; Sahoo, A.K. Silver(I)-Catalyzed Reaction between Pyrazole and Propargyl Acetates: Stereoselective Synthesis of the Scorpionate Ligands (E)-Allyl-gem-dipyrazoles (ADPs). J. Org. Chem. Res. 2013, 78, 11824. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Tomilin, D.N.; Petrova, O.V.; Sobenina, L.N.; Mikhaleva, A.I.; Trofimov, B.A. A convenient synthesis of hetarylethynyl ketones from hetarylcarbaldehydes and acetylene. Chem. Heterocycl. Compd. 2013, 49, 341–344. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gotsko, M.D.; Saliy, I.V.; Vashchenko, A.V.; Trofimov, B.A. 1-Phenyl-3,3-di(1H-pyrazol-1-yl)propan-1-one. Molbank 2022, 2022, M1472. https://doi.org/10.3390/M1472
Gotsko MD, Saliy IV, Vashchenko AV, Trofimov BA. 1-Phenyl-3,3-di(1H-pyrazol-1-yl)propan-1-one. Molbank. 2022; 2022(4):M1472. https://doi.org/10.3390/M1472
Chicago/Turabian StyleGotsko, Maxim D., Ivan V. Saliy, Alexander V. Vashchenko, and Boris A. Trofimov. 2022. "1-Phenyl-3,3-di(1H-pyrazol-1-yl)propan-1-one" Molbank 2022, no. 4: M1472. https://doi.org/10.3390/M1472
APA StyleGotsko, M. D., Saliy, I. V., Vashchenko, A. V., & Trofimov, B. A. (2022). 1-Phenyl-3,3-di(1H-pyrazol-1-yl)propan-1-one. Molbank, 2022(4), M1472. https://doi.org/10.3390/M1472





