(E)-1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)-3-(naphthalen-2-yl)prop-2-en-1-one
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of 3
2.2. NMR Spectroscopy
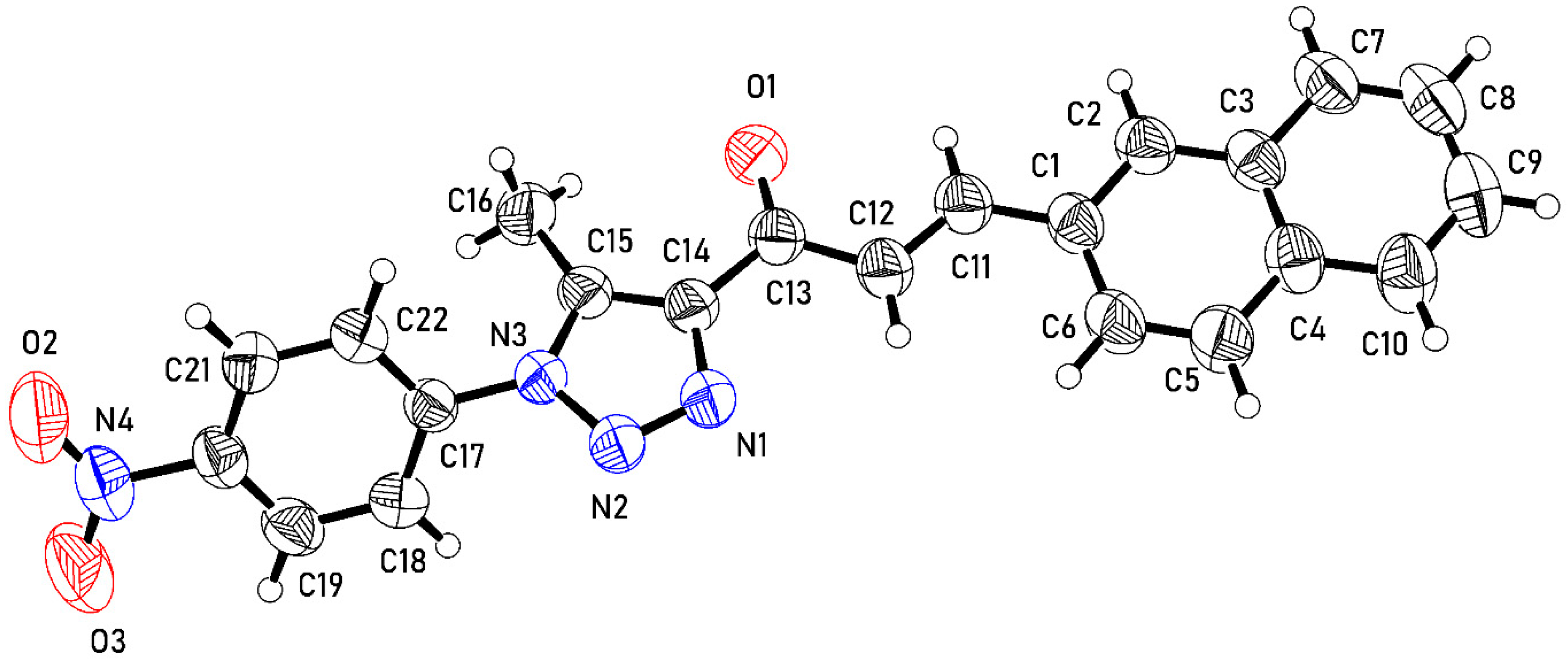
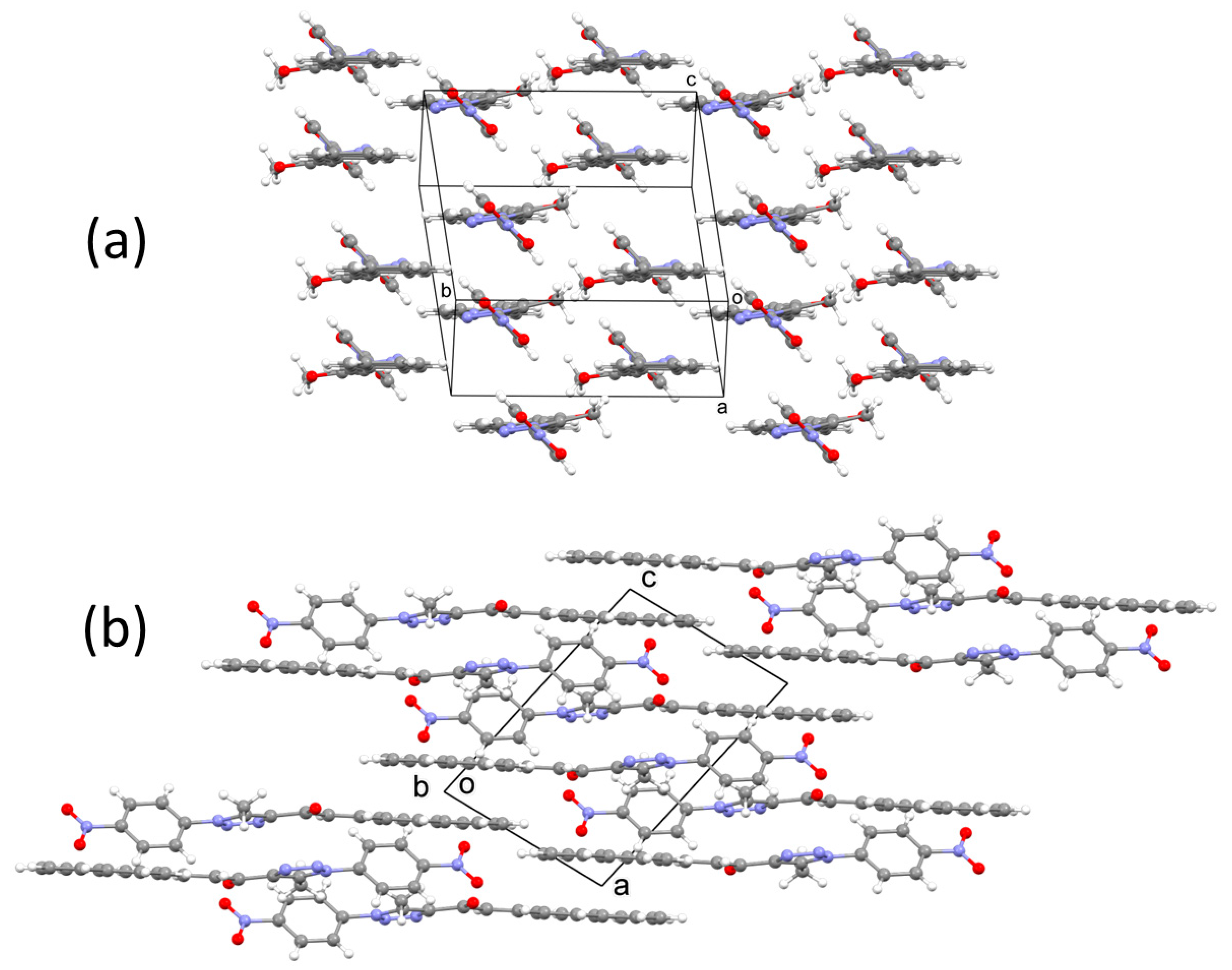
2.3. X-ray Crystal Structure
3. Materials and Methods
3.1. General
3.2. Synthesis of 3
3.3. Data Collection and Structure Refinement Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Królicka, E.; Kieć-Kononowicz, K.; Łażewska, D. Chalcones as Potential Ligands for the Treatment of Parkinson’s Disease. Pharmaceuticals 2022, 15, 847. [Google Scholar] [CrossRef]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef]
- Bonvicini, F.; Gentilomi, G.A.; Bressan, F.; Gobbi, S.; Rampa, A.; Bisi, A.; Belluti, F. Functionalization of the Chalcone Scaffold for the Discovery of Novel Lead Compounds Targeting Fungal Infections. Molecules 2019, 24, 372. [Google Scholar] [CrossRef]
- Jesus, A.; Durães, F.; Szemerédi, N.; Freitas-Silva, J.; da Costa, P.M.; Pinto, E.; Pinto, M.; Spengler, G.; Sousa, E.; Cidade, H. BDDE-inspired chalcone derivatives to fight bacterial and fungal infections. Mar. Drugs 2022, 20, 315. [Google Scholar] [CrossRef]
- George, G.; Koyiparambath, V.P.; Sukumaran, S.; Nair, A.S.; Pappachan, L.K.; Al-Sehemi, A.G.; Kim, H.; Mathew, B. Structural modifications on chalcone framework for developing new class of cholinesterase inhibitors. Int. J. Mol. Sci. 2022, 23, 3121. [Google Scholar] [CrossRef] [PubMed]
- Mendanha, D.; Vieira de Castro, J.; Moreira, J.; Costa, B.M.; Cidade, H.; Pinto, M.; Ferreira, H.; Neves, N.M. A new chalcone derivative with promising antiproliferative and anti-Invasion activities in glioblastoma cells. Molecules 2021, 26, 3383. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring pharmacological significance of chalcone scaffold: A review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone derivatives: Role in anticancer therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Matos, M.J.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L. Potential pharmacological uses of chalcones: A patent review (from June 2011–2014). Expert Opin. Ther. Pat. 2015, 25, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Lungu, C.N. Anticancer activity of natural and synthetic chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef]
- Gao, F.; Huang, G.; Xiao, J. Chalcone hybrids as potential anticancer agents: Current development, mechanism of action and structure-activity relationship. Med. Res. Rev. 2020, 40, 2049–2084. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.; Ahmed, K.; Padhye, S.; Ahmad, A.; Biersack, B. Anticancer active heterocyclic chalcones: Recent developments. Anticancer Agents Med. Chem. 2021, 21, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Pathak, D.P.; Bansal, H.; Agarwal, U. Chalcone and their heterocyclic analog: A review article. J. Chem. Pharm. Res. 2018, 10, 160–173. [Google Scholar]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2000, 18, 433–458. [Google Scholar] [CrossRef]
- Qian, H.; Liu, D.; Lv, C. Synthesis of chalcones via Claisen-Schmidt reaction catalyzed by sulfonic acid-functional ionic liquids. Ind. Eng. Chem. Res. 2011, 50, 1146–1149. [Google Scholar] [CrossRef]
- Ekanayake, U.G.M.; Weerathunga, H.; Weerasinghe, J.; Waclawik, E.R.; Sun, Z.; MacLeod, J.M.; O‘Mullane, A.P.; Ostrikov, K. Sustainable Claisen-Schmidt chalcone synthesis catalysed by plasma-recovered MgO nanosheets from seawater. Sustain. Mater. Technol. 2022, 32, e00394. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Sastry, V.G.; Bano, N.; Anwar, S. Synthesis of novel chalcone derivatives by conventional and microwave irradiation methods and their pharmacological activities. Arab. J. Chem. 2016, 9, S931–S935. [Google Scholar] [CrossRef]
- Lannes, A.C.; Leal, B.; Novais, J.S.; Lione, V.; Monteiro, G.C.T.S.; Lourenço, A.L.; Sathler, P.C.; Jordão, A.K.; Rodrigues, C.R.; Cabral, L.M.; et al. Exploring N-acylhydrazone derivatives against clinical resistant bacterial strains. Curr. Microbiol. 2014, 69, 357–364. [Google Scholar] [CrossRef] [PubMed]
- He, J.-B.; Feng, L.-L.; Li, J.; Tao, R.-J.; Ren, Y.-L.; Wan, J.; He, H.-W. Design, synthesis and molecular modeling of novel N-acylhydrazone derivatives as pyruvate dehydrogenase complex E1 inhibitors. Bioorg. Med. Chem. 2014, 22, 89–94. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Alotaibi, M.H.; El-Hiti, G.A. Synthesis of new symmetrical N,N’-diacylhydrazines and 2-(1,2,3-triazol-4-yl)-1,3,4-oxadiazoles. Lett. Org. Chem. 2017, 14, 591–596. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Khidre, R.E.; Kariuki, B.M.; El-Hiti, G.A. Synthesis of novel heterocycles using 1,2,3-triazole-4-carbohydrazides as precursors. J. Heterocycl. Chem. 2020, 57, 1055–1062. [Google Scholar] [CrossRef]
- Jadhav, R.P.; Raundal, U.N.; Patil, A.A.; Bobade, V.D. Synthesis and biological evaluation of a series of 1,4-disubstituted 1,2,3-triazole derivatives as possible antimicrobial agents. J. Saudi Chem. Soc. 2017, 21, 152–159. [Google Scholar] [CrossRef]
- Krishna, P.M.; Ramachary, D.B.; Peesapati, S. Azide–acetonitrile “click” reaction triggered by Cs2CO3: The atom-economic, high-yielding synthesis of 5-amino-1,2,3-triazoles. RSC Adv. 2015, 5, 62062–62066. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Matiychuk, V.S.; Obushak, N.B. Synthesis of 1H-1,2,3-triazole derivatives by the cyclization of aryl azides with 2-benzothiazolylacetonone, 1,3-benzo-thiazol-2-ylacetonitrile, and (4-aryl-1,3-thiazol-2-yl) acetonitriles. Chem. Heterocycl. Compd. 2009, 45, 483–488. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Liu, G.; Xu, H.; Song, L.; Chen, J.; Li, J.; Zhang, Z. Transition-metal-free synthesis of 5-amino-1,2,3-triazoles via nucleophilic addition/cyclization of carbodiimides with diazo compounds. Org. Chem. Front. 2021, 8, 599–604. [Google Scholar] [CrossRef]
- Opsomer, T.; Thomas, J.; Dehaen, W. Chemoselectivity in the synthesis of 1,2,3-triazoles from enolizable ketones, primary alkylamines, and 4-nitrophenyl azide. Synthesis 2017, 49, 4191–4198. [Google Scholar] [CrossRef]
- Duan, H.; Sengupta, S.; Petersen, J.L.; Akhmedov, N.G.; Shi, X. Triazole-Au(I) complexes: A new class of catalysts with improved thermal stability and reactivity for intermolecular alkyne hydroamination. J. Am. Chem. Soc. 2009, 131, 12100–12102. [Google Scholar] [CrossRef]
- Gribanov, P.S.; Atoian, E.M.; Philippova, A.N.; Topchiy, M.A.; Asachenko, A.F.; Osipov, S.N. One-pot synthesis of 5-amino-1,2,3-triazole derivatives via dipolar azide–nitrile cycloaddition and Dimroth rearrangement under solvent-free conditions. Eur. J. Org. Chem. 2021, 2021, 1378–1384. [Google Scholar] [CrossRef]
- Gribanov, P.S.; Topchiy, M.A.; Karsakova, I.V.; Chesnokov, G.A.; Smirnov, A.Y.; Minaeva, L.I.; Asachenko, A.F.; Nechaev, M.S. General method for the synthesis of 1,4-disubstituted 5-halo-1,2,3-triazoles. Eur. J. Org. Chem. 2017, 2017, 5225–5230. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Farahat, A.A.; El-Hiti, G.A. Synthesis and structure determination of 1-(4-methoxyphenyl)-5-methyl-N’-(2-oxoindolin-3-ylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molbank 2022, 2022, M1374. [Google Scholar] [CrossRef]
- Gökce, H.; Şen, F.; Sert, Y.; Abdel-Wahab, B.F.; Kariuki, B.M.; El-Hiti, G.A. Quantum computational investigation of (E)-1-(4-methoxyphenyl)-5-methyl-N’-(3-phenoxybenzylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molecules 2022, 27, 2193. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, B.M.; Abdel-Wahab, B.F.; El-Hiti, G.A. Synthesis and structural characterization of isostructural 4-(4-aryl)-2-(5-(4-fluorophenyl)-3-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles. Crystals 2021, 11, 795. [Google Scholar] [CrossRef]
- Alotaibi, M.H.; Mohamed, H.A.; Abdel-Wahab, B.F.; Hegazy, A.S.; Kariuki, B.M.; El-Hiti, G.A. Synthesis and structure elucidation of N’-(4-methoxybenzylidene)-5-methyl-1-phenyl-1H-1,2,3-triazole-4-carbohydrazide. Molbank 2018, 2018, M1034. [Google Scholar] [CrossRef]
- Kamalraj, V.R.; Senthil, S.; Kannan, P. One-pot synthesis and the fluorescent behavior of 4-acetyl-5-methyl-1,2,3-triazole regioisomers. J. Mol. Struct. 2008, 892, 210–215. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
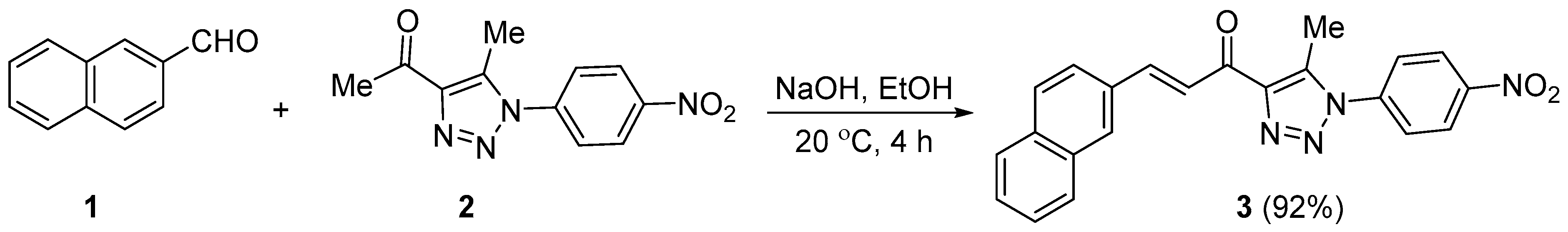
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariuki, B.M.; Abdel-Wahab, B.F.; Mohamed, H.A.; El-Hiti, G.A. (E)-1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)-3-(naphthalen-2-yl)prop-2-en-1-one. Molbank 2022, 2022, M1464. https://doi.org/10.3390/M1464
Kariuki BM, Abdel-Wahab BF, Mohamed HA, El-Hiti GA. (E)-1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)-3-(naphthalen-2-yl)prop-2-en-1-one. Molbank. 2022; 2022(4):M1464. https://doi.org/10.3390/M1464
Chicago/Turabian StyleKariuki, Benson M., Bakr F. Abdel-Wahab, Hanan A. Mohamed, and Gamal A. El-Hiti. 2022. "(E)-1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)-3-(naphthalen-2-yl)prop-2-en-1-one" Molbank 2022, no. 4: M1464. https://doi.org/10.3390/M1464
APA StyleKariuki, B. M., Abdel-Wahab, B. F., Mohamed, H. A., & El-Hiti, G. A. (2022). (E)-1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)-3-(naphthalen-2-yl)prop-2-en-1-one. Molbank, 2022(4), M1464. https://doi.org/10.3390/M1464







