(Diaqua)hexakis(4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dionato-κ2O,O’)(μ-(1,4-dioxane))digadolinium (III), Solvate with Two Molecules of 1,4-Dioxane
Abstract
:1. Introduction
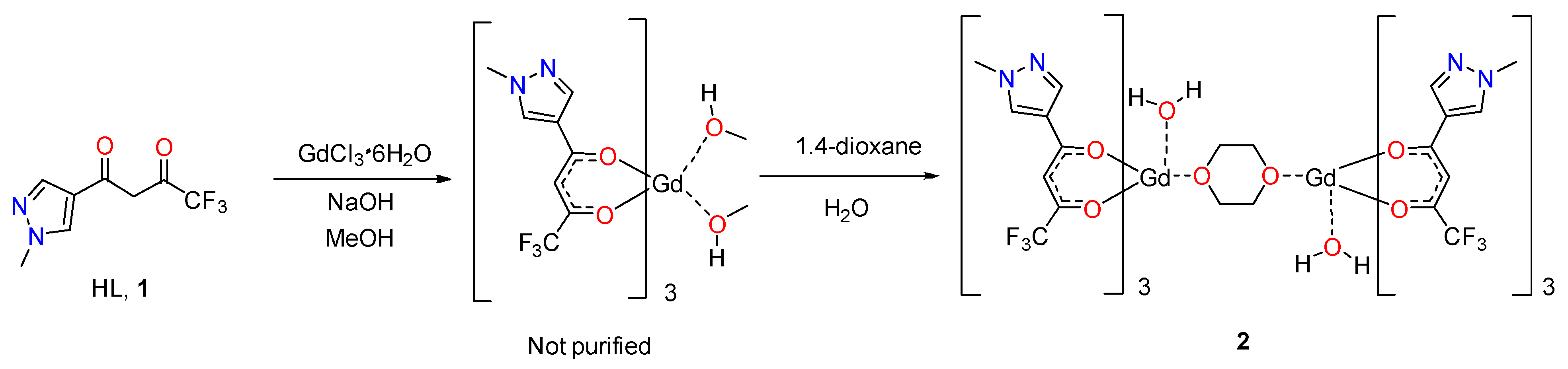
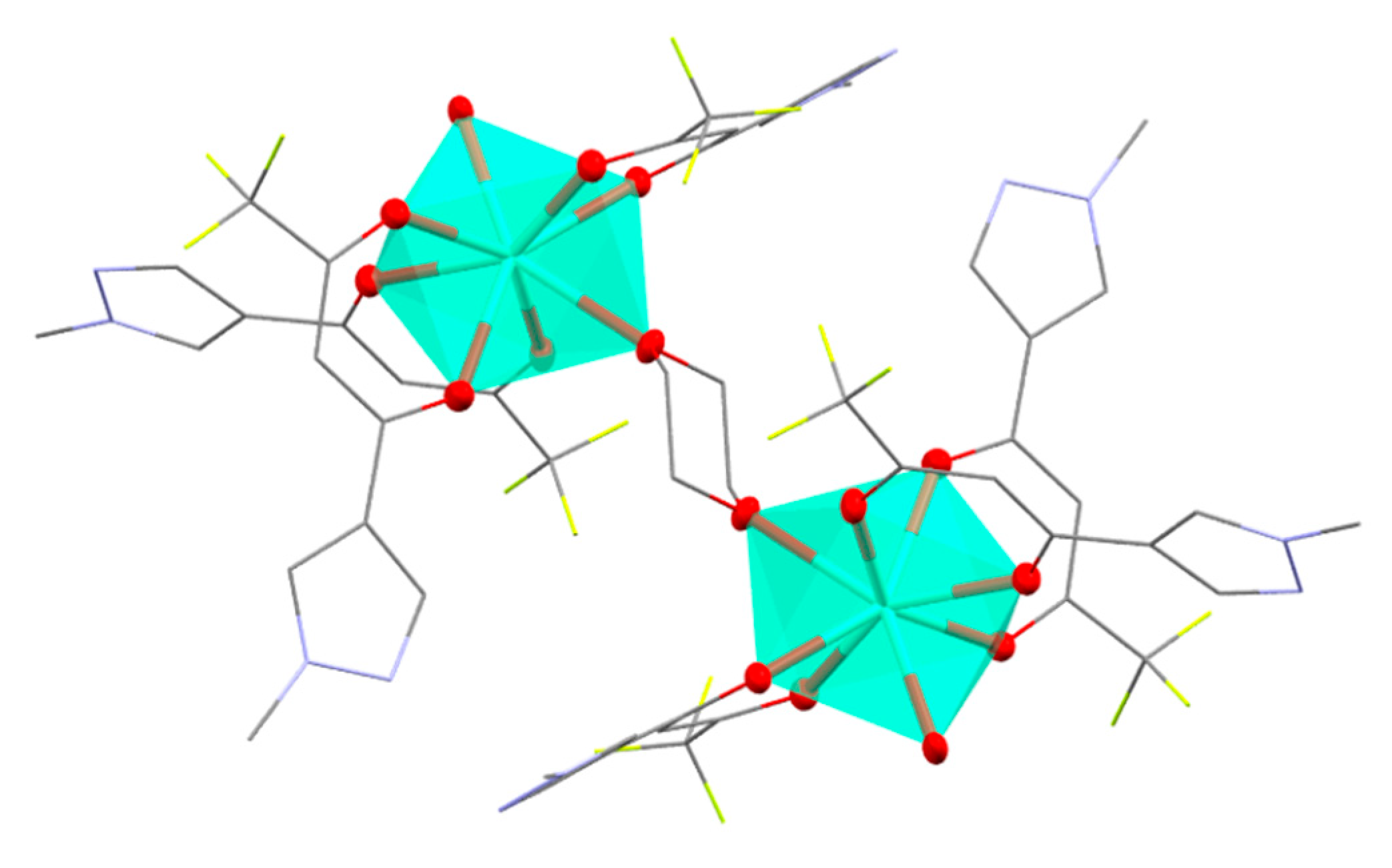
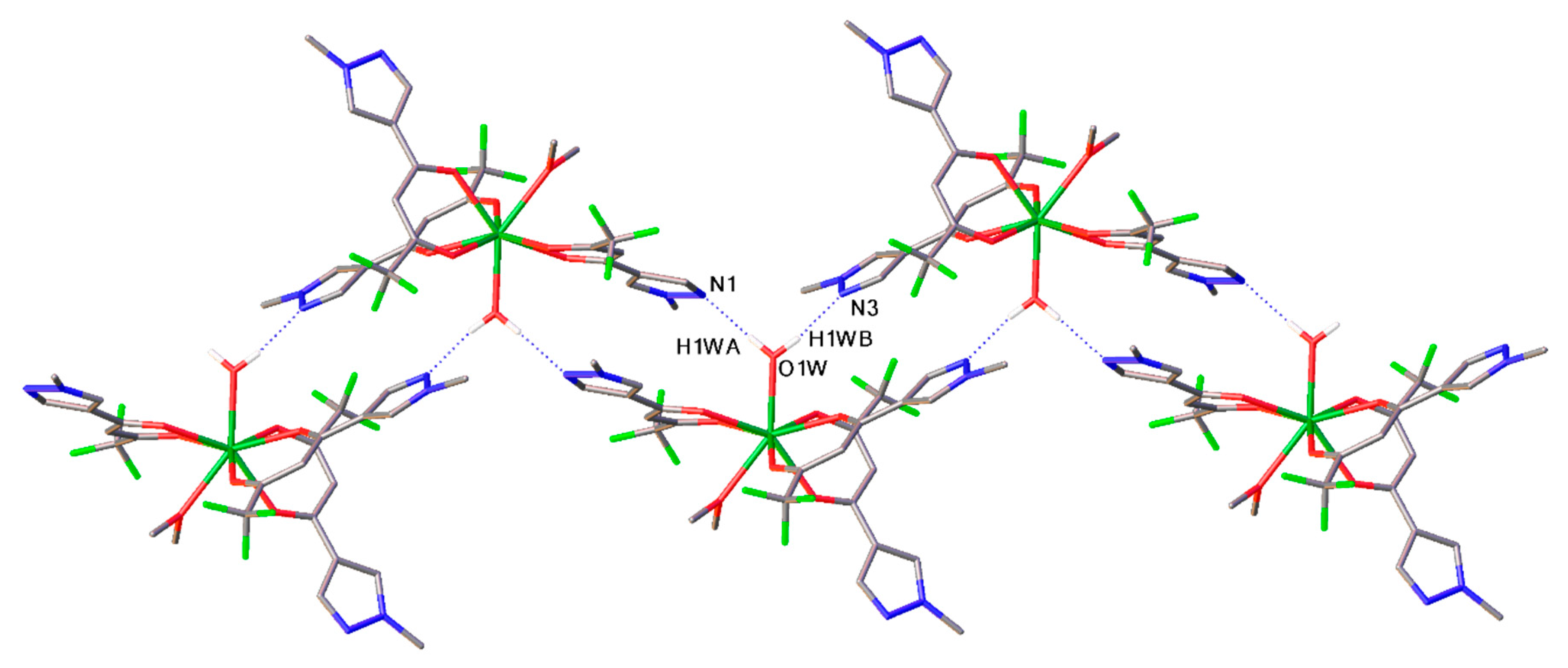
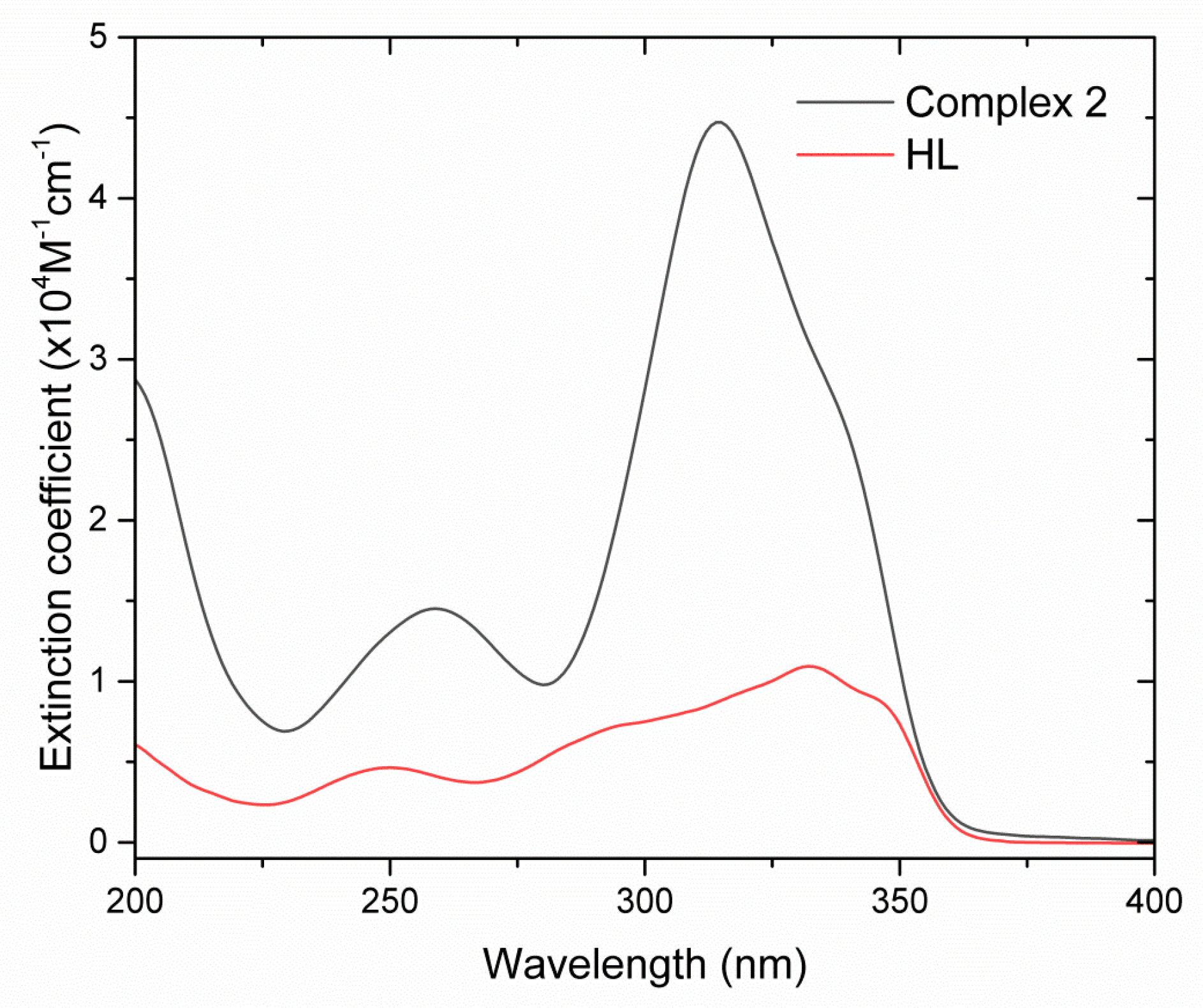
2. Results and Discussion
3. Materials and Methods
Synthesis of (Diaqua)hexakis(4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dionato-κ2O,O’)(μ-(1,4-dioxane))digadolinium (III), Solvate with Two Molecules of 1,4-Dioxane, Complex 2
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metlina, D.A.; Metlin, M.T.; Ambrozevich, S.A.; Taydakov, I.V.; Lyssenko, K.A.; Vitukhnovsky, A.G.; Selyukov, A.S.; Krivobok, V.S.; Aminev, D.F.; Tobokhova, A.S. Luminescence and Electronic Structure of Nd3+ Complex with Pyrazole-Substituted 1,3-Diketone and 1,10-Phenanthroline. J. Lumin. 2018, 203, 546–553. [Google Scholar] [CrossRef]
- Sizov, V.S.; Komissar, D.A.; Metlina, D.A.; Aminev, D.F.; Ambrozevich, S.A.; Nefedov, S.E.; Varaksina, E.A.; Metlin, M.T.; Mislavskií, V.V.; Taydakov, I.V. Effect of Ancillary Ligands on Visible and NIR Luminescence of Sm3+ β-Diketonate Complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117503. [Google Scholar] [CrossRef] [PubMed]
- Metlina, D.A.; Metlin, M.T.; Ambrozevich, S.A.; Selyukov, A.S.; Datskevich, N.P.; Aminev, D.F.; Goryachii, D.O.; Lyssenko, K.A.; Pavlov, A.A.; Dmitrienko, A.O.; et al. Bright NIR-Luminescent Nd3+ Complexes with Pyrazole-Substituted 1,3-Diketones Demonstrated an Unusual Spectral Lines Branching Ratios. Dye. Pigment. 2020, 181, 108558. [Google Scholar] [CrossRef]
- Taidakov, I.; Krasnosel’skii, S.; Lobanov, A.; Vitukhnovskii, A.; Starikova, Z. Cesium Tetrakis(1-(1,5-Dimethyl-1H-Pyrazol-4-Yl)-4,4,4-Trifluorobutane-1,3-Diono)Europiate(III): Synthesis, Crystal Structure, and Luminescence Properties. Russ. J. Coord. Chem. 2013, 39, 680–684. [Google Scholar] [CrossRef]
- Taidakov, I.; Lobanov, A.; Vitukhnovskii, A.; Starikova, Z. Synthesis and Unusual Crystal Structure of the Eu(III) Complex with 1-(1,5-Dimethyl-1H-Pyrazol-4-Yl)-4,4,4-Trifluorobutane-1,3-Dione. Russ. J. Coord. Chem. 2013, 39, 437–441. [Google Scholar] [CrossRef]
- Gontcharenko, V.; Kiskin, M.; Dolzhenko, V.; Korshunov, V.; Taydakov, I.; Belousov, Y. Mono- and Mixed Metal Complexes of Eu3+, Gd3+, and Tb3+ with a Diketone, Bearing Pyrazole Moiety and CHF2-Group: Structure, Color Tuning, and Kinetics of Energy Transfer between Lanthanide Ions. Molecules 2021, 26, 2655. [Google Scholar] [CrossRef] [PubMed]
- Trannoy, V.; Carneiro Neto, A.N.; Brites, C.D.S.; Carlos, L.D.; Serier-Brault, H. Engineering of Mixed Eu3+/Tb3+ Metal-Organic Frameworks Luminescent Thermometers with Tunable Sensitivity. Adv. Opt. Mater. 2021, 9, 2001938. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Rising Stars in Science and Technology: Luminescent Lanthanide Materials: Rising Stars in Science and Technology: Luminescent Lanthanide Materials. Eur. J. Inorg. Chem. 2017, 2017, 5058–5063. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A.; Taydakov, I.V.; Marchetti, F.; Pettinari, R.; Pettinari, C. Lanthanide Azolecarboxylate Compounds: Structure, Luminescent Properties and Applications. Coord. Chem. Rev. 2021, 445, 214084. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Basics of Lanthanide Photophysics. In Lanthanide Luminescence; Hänninen, P., Härmä, H., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2010; Volume 7, pp. 1–45. ISBN 978-3-642-21022-8. [Google Scholar]
- Metlin, M.T.; Goryachii, D.O.; Aminev, D.F.; Datskevich, N.P.; Korshunov, V.M.; Metlina, D.A.; Pavlov, A.A.; Mikhalchenko, L.V.; Kiskin, M.A.; Garaeva, V.V.; et al. Bright Yb3+ Complexes for Efficient Pure Near-Infrared OLEDs. Dye. Pigment. 2021, 195, 109701. [Google Scholar] [CrossRef]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Taydakov, I.V.; Krasnoselsky, S.S. Modified Method for the Synthesis of Isomeric N-Substituted (1H-Pyrazolyl)Propane-1,3-Diones. Chem. Heterocycl. Compd. 2011, 47, 695. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. A Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]



| Square Antiprism, D4d | Triangular Dodecahedron, D2d | Biaugmented Trigonal Prism J50, C2v | Biaugmented Trigonal Prism, C2v | Snub Diphenoid J84, D2d |
|---|---|---|---|---|
| 0.687 | 1.124 | 2.303 | 1.609 | 3.603 |
| Bond | Distance, Å |
|---|---|
| Gd1-O1 | 2.359(2) |
| Gd1-O2 | 2.372(2) |
| Gd1-O3 | 2.335(2) |
| Gd1-O4 | 2.382(2) |
| Gd1-O5 | 2.345(2) |
| Gd1-O6 | 2.348(2) |
| Gd1-O7 | 2.490(2) |
| Gd1-O1W | 2.380(2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taydakov, I.V.; Belousov, Y.A.; Metlin, M.T.; Gontcharenko, V.E. (Diaqua)hexakis(4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dionato-κ2O,O’)(μ-(1,4-dioxane))digadolinium (III), Solvate with Two Molecules of 1,4-Dioxane. Molbank 2022, 2022, M1452. https://doi.org/10.3390/M1452
Taydakov IV, Belousov YA, Metlin MT, Gontcharenko VE. (Diaqua)hexakis(4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dionato-κ2O,O’)(μ-(1,4-dioxane))digadolinium (III), Solvate with Two Molecules of 1,4-Dioxane. Molbank. 2022; 2022(4):M1452. https://doi.org/10.3390/M1452
Chicago/Turabian StyleTaydakov, Ilya V., Yury A. Belousov, Mikhail T. Metlin, and Victoria E. Gontcharenko. 2022. "(Diaqua)hexakis(4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dionato-κ2O,O’)(μ-(1,4-dioxane))digadolinium (III), Solvate with Two Molecules of 1,4-Dioxane" Molbank 2022, no. 4: M1452. https://doi.org/10.3390/M1452
APA StyleTaydakov, I. V., Belousov, Y. A., Metlin, M. T., & Gontcharenko, V. E. (2022). (Diaqua)hexakis(4,4,4-trifluoro-1-(1-methyl-1H-pyrazol-4-yl)butane-1,3-dionato-κ2O,O’)(μ-(1,4-dioxane))digadolinium (III), Solvate with Two Molecules of 1,4-Dioxane. Molbank, 2022(4), M1452. https://doi.org/10.3390/M1452






