Abstract
Cyclic adenosine diphosphate ribose (cADPR) is a cyclic nucleotide involved in the Ca2+ homeostasis. In its structure, the northern ribose, bonded to adenosine through an N1 glycosidic bond, is connected to the southern ribose through a pyrophosphate bridge. Due to the chemical instability at the N1 glycosidic bond, new bioactive cADPR derivatives have been synthesized. One of the most interesting analogues is the cyclic inosine diphosphate ribose (cIDPR), in which the hypoxanthine replaced adenosine. The efforts for synthesizing new linear and cyclic northern ribose modified cIDPR analogues led us to study in detail the inosine N1 alkylation reaction. In the last few years, we have produced new flexible cIDPR analogues, where the northern ribose has been replaced by alkyl chains. With the aim to obtain the closest flexible cIDPR analogue, we have attached to the inosine N1 position a 2″,3″-dihydroxypentyl chain, possessing the two OH groups in a ribose-like fashion. The inosine alkylation reaction afforded also the O6-alkylated regioisomer, which could be a useful intermediate for the construction of new kinds of cADPR mimics.
1. Introduction
The design and synthesis of new nucleoside and nucleotide analogues is a frontier theme in light of the current SARS-CoV-2 pandemic that the world is facing [1]. However, apart from being employed in medicinal chemistry both as antiviral [2] and antitumor drugs [3], nucleosides, nucleotides, and their analogues can be also used as probes in the signaling pathways. [4,5,6] Cyclic nucleotides are important second messengers involved in signal transduction. [7,8] Among them, cADPR (1, Figure 1), an 18-membered cyclic nucleotide firstly isolated from sea urchin egg extracts [9], elicits Ca2+ ions from the endoplasmic reticulum (ER) to cytosol through the ryanodine receptor (RyR) in several cellular systems [10]. Alterations in the cADPR biosynthesis and calcium homeostasis can play pathological roles in diabetes, airway hyper-responsiveness and autism [11]. Unfortunately, the chemical instability at the N1 glycosidic bond in physiological conditions hampered the definition of cADPR molecular mechanism of action. [12] In this frame, efforts have been devoted to the synthesis of stable cADPR analogues [13,14,15,16]. In particular, the replacement of the adenine with hypoxanthine generated the very stable cIDPR analogue 2, which retained the same Ca2+ mobilizing activity of the endogenous metabolite [17]. In the last few years, we have produced both in solution [18,19,20,21] and on solid phase [22,23] some cIDPR analogues with alkyl chains in the place of the northern ribose (3–7). As we have found a promising Ca2+ mobilizing activity in the derivative with a pentyl chain (6, n = 4) in a neuronal cellular model [18], we have recently prepared a new flexible cIDPR analogue (8), with a 2″,3″-dihydroxypentyl chain replacing the northern ribose [21].
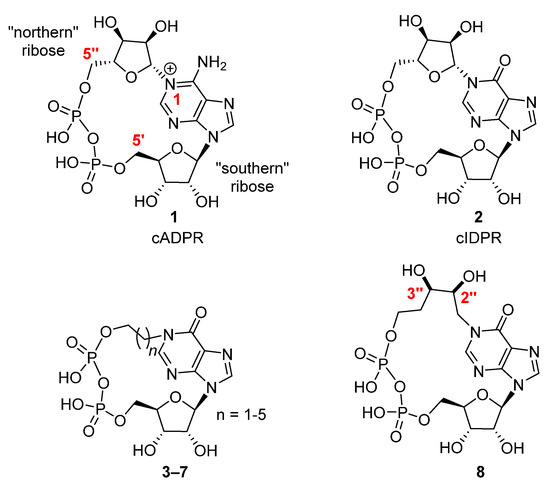
Figure 1.
The structures of cADPR (1), cIDPR (2) and their analogues (3–8).
As the last mimic possesses the two OH groups in a ribose-like fashion, it may be considered the closest cIDPR flexible analogue. Interestingly, the cyclic compound 8 induced a concentration-dependent increase in [Ca2+]i when perfused to primary cortical neurons as efficiently as cADPR.
Since 6-substituted purine nucleosides displayed interesting biological activities [24,25,26], herein we report on the synthesis and characterization of the O6-alkylated compound 9, obtained as a side product during the coupling reaction of the protected inosine 10 and the tosylate 11 (Scheme 1). That reaction was revealed to be fundamental for the construction of the new cyclic analogue (8) scaffold.
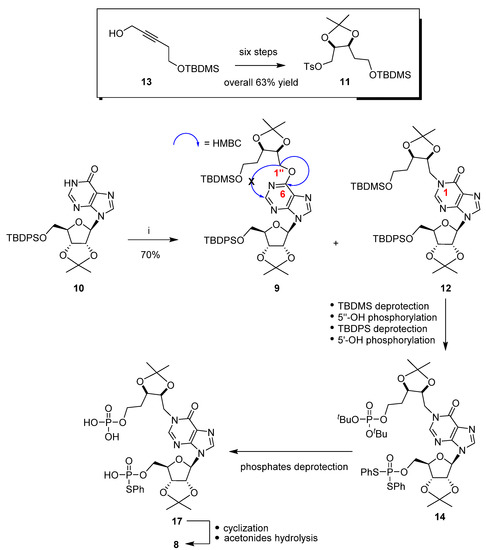
Scheme 1.
Reagents and conditions: (i) 11, DBU, DMF, 80 °C, 12 h (entry 6, Table 1).
2. Results and Discussion
Scheme 1 shows the convergent synthetic approach that we have used for the preparation of the cyclic compound 8. The ribose-protected inosine 10, after reaction with the tosylate 11, afforded as the main product the N1-alkylated derivative 12. The electrophile 11 was prepared as a racemic mixture starting from the commercially available propargyl alcohol 13 with an overall 63% yield [21].
To obtain the target compound 8, we used the well-known Hata protocol, which required an I2 mediated cyclopyrophosphorylation reaction among a phosphomonoester and a phosphorothioate [14]. As a TBDMS group could be selectively removed in the presence of a TBDPS group [27], we followed the synthetic sequence reported in Scheme 1, and compound 14 was readily obtained. The two phosphates in 14 were then deprotected to give the key intermediate 17. The pyrophosphate bond formation was carried out by adding compound 17 through a syringe pump to a very diluted solution of I2. The cyclic compound was isolated from a complex reaction mixture and finally deprotected from both the acetonides, affording the new cADPR analogue 8 with an overall 1% yield.
The reaction between the protected inosine 10 with the electrophile 11 has been studied in detail. In principle, as hypoxanthine is an ambident nucleophile [28] at the N1 and O6 purine positions [29,30], the two regioisomers 12 and 9 could be expected. In our studies focused on the inosine N1 functionalization, we discovered that the Mitsunobu reaction was not a good method to regioselectivity obtain the N1-alkylated isomer. In fact, the reaction of inosine with di-tert-butyl (5-hydroxypentyl)phosphonate in the presence of diethyl azodicarboxylate (DEAD) and triphenylphosphine (PPh3) afforded mainly the O6-alkylated regioisomer. We attributed the observed regioselectivity to the hard–hard interaction between the alkoxy-phosphonium cation intermediate and the nucleobase O6 atom. [19] On the other hand, previous findings demonstrated that inosine could be efficiently alkylated at the N1 purine position at room temperature (r.t.) when a soft electrophile was used. [31] Unfortunately, all the attempts to transform both the tosylate 11 and its primary alcohol precursor into the iodide derivative proceeded with very unsatisfactory yields. [21] As literature data reported on the efficient nucleophilic mediated displacement of a tosylate flanked by an isopropylidene under mild conditions [32,33], we reacted the inosine derivative 10 with the electrophile 11 at r.t. in the presence of some bases (Table 1); unfortunately, no reaction took place. By increasing the temperature, we noted on TLC (petroleum ether/AcOEt, 6:4) the formation of two spots. 1D/2D NMR analyses of the purified compounds (see Supplementary Materials) allowed to assign the N1-alkylated inosine 12 to the spot with Rf = 0.10, whereas the O6-alkylated inosine 9 to that with Rf = 0.50. In detail, in the 1H NMR spectrum of compound 12 the two 1″-H protons resonated as two doublets of doublets centered at 4.70 and 4.66 ppm and correlated with their carbon atom resonating around 47.7 ppm. The HMBC correlation between the purine 2-H and the C1″ carbon atom supported the structure 12. [21] Conversely, in the 1H NMR spectrum of compound 9 (Figure S1, Supplementary Materials) the 1″-H protons resonated in the range 4.68–4.54 ppm and correlated with their carbon atom resonating at 65.6 ppm (Figure S4). The HMBC correlation between the two 1″-H protons and the C6 purine carbon atom resonating at 160.3 ppm and the absence of HMBC correlation between the purine 2-H (8.43 ppm) and the C1″ carbon atom supported the structure 9 (Figure S5).

Table 1.
Optimization of the reaction between 10 and 11 1.
Careful tuning of the reaction conditions allowed to recover the N1-alkylated regioisomer 12 as the main product. In particular, 3.0 equiv. of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 80 °C for 12 h were the best conditions to recover the N1 and O6 regioisomers with a good 70% yield in a 7:3 ratio. (Scheme 2 and Table 1) Higher temperatures were detrimental to the reaction yield and increased the ratio 9:12. The last experimental evidence could be explained assuming that compound 9 is the thermodynamic regioisomer.
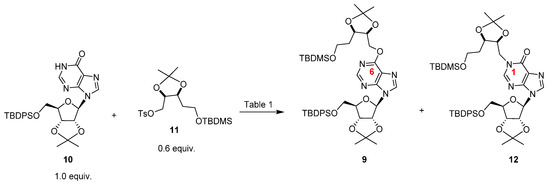
Scheme 2.
The reaction of the nucleoside 10 with the tosylate 11.
3. Materials and Methods
All the reagents and solvents were commercially available and used without further purification. 1H- and 13C-NMR spectra were acquired on the Bruker Avance 600 MHz spectrometer (Bruker-Biospin, Billerica, MA, USA) using CDCl3 as solvent. NMR chemical shifts are reported in parts per million (δ) relative to residual solvents signals: CHCl3 7.26 for 1H-NMR and CDCl3 77.0, for 13C-NMR. The 1H NMR chemical shifts were assigned through 2D NMR experiments. The NMR spectra were processed with the MestReNova (Mestrelab Research, Santiago de Campostela, Spain) suite. The HRESI-MS spectra were acquired on a Thermo Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Column chromatography was carried out on silica gel-60 (Merck, Readington Township, NJ, USA, 0.063–0.200 mm). TLC analyses were carried out on F254 silica gel plates (0.2 mm thick, Merck). TLC spots were detected under UV light (254 nm).
O6-[(2″,3″-O-isopropylidene-5″-O-tert-butyldimethylsilyl)pentyl]-5′-O-tert-butyldiphenylsilyl-2′,3′-O-isopropylideneinosine 9. To a stirred solution of compound 10 (0.10 g, 0.18 mmol) in anhydrous DMF (2.0 mL), DBU (80 µL, 0.55 mmol) was added. After 20 min. the tosylate 11 (49 mg, 0.11 mmol) was added and reaction warmed to 80 °C for 12 h (TLC monitoring: petroleum ether/AcOEt, 6:4). The mixture was cooled, and the solvents removed under reduced pressure. The crude product was purified over a silica gel column eluted with increasing amounts of AcOEt in petroleum ether (up to 20%), giving the pure 9 as a 1:1 mixture of diastereomers (Rf = 0.50). Colorless syrup (21% yield). 1H NMR (600 MHz, CD3Cl) δ 8.43 (s, 1H, 2-H), 8.42 (s, 1H, 2-H), 8.07 (s, 1H, 8-H), 8.07 (s, 1H, 8-H), 7.62−7.54 (complex signal, 8H, arom.), 7.43−7.25 (complex signal, 12H, arom.), 6.15 (d, J = 1.7 Hz, 2H, 2 × 1′-H), 5.32−5.28 (m, 2H, 2 × 2′-H), 4.98−4.95 (m, 2H, 2 × 3′-H), 4.68−4.62 (m, 2H, 2 × 1″-Ha), 4.61−4.54 (complex signal, 4H, 2 × 1″-Hb and 2 × 2″-H), 4.49−4.43 (m, 2H, 2 × 3″-H), 4.42−4.38 (m, 2H, 2 × 4′-H), 3.92−3.85 (m, 2H, 2 × 5′-Ha), 3.83−3.74 (complex signal, 6H, 2 × 5′-Hb and 2 × 5″-Ha,b), 1.97−1.89 (m, 2H, 2 × 4″-Ha), 1.85−1.75 (m, 2H, 2 × 4″-Hb), 1.62 (s, 6H, 2 × CH3 acetonide), 1.48 (s, 6H, 2 × CH3 acetonide), 1.37 (two overlapped singlets, 12H, 4 × CH3 acetonide), 1.01 (two overlapped singlets, 18H, 2 × tBu), 0.88 (s, 18H, 2 × tBu), 0.04 (s, 6H, 2 × SiCH3), 0.05 (s, 6H, 2 × SiCH3). 13C NMR (151 MHz, CDCl3) δ 160.32 (2 × C6), 152.10 (C2), 152.08 (C2), 151.45 (C4), 151.44 (C4), 141.01 (C8), 140.98 (C8), 135.49, 135.47, 132.82, 132.80, 132.69, 129.85, 127.74, 127.69, 127.62, 122.05 (2 × C5), 114.34 (Cq ribose acetonide) 114.33 (Cq ribose acetonide), 108.37 (2 × Cq acetonide), 91.19 (C1′), 91.17 (C1′), 87.02 (C4′), 86.97 (C4′), 84.40 (C2′), 84.36 (C2′), 81.36 (C3′), 81.35 (C3′), 75.14 (C2″), 75.12 (C2″), 73.71 (C3″), 73.70 (C3″), 65.60 (2 × C1″), 63.85 (2 × C5′), 60.02 (C5″), 60.00 (C5″), 32.16 (C4″), 32.14 (C4″), 29.66, 28.19, 28.17, 27.19, 26.81, 25.91, 25.86, 25.58, 25.35, 19.15, 18.30, −5.39, −5.44. HRESI-MS m/z 819.4184, ([M + H]+ calcd. for C43H63N4O8Si2 819.4191).
4. Conclusions
Purine bases and nucleosides carrying O-, N- and C-substituents at the C6 position represent an important class of compounds endowed with important biological activities. These compounds are generally prepared by nucleophilic aromatic (SNAr) substitutions [34], metal-mediated cross-coupling reactions [35,36] and by Grignard’s reagents addition [26] to 6-halopurine ribosides. In our search of new cADPR analogues, we have obtained the O6-alkylated inosine 9 as a side product during the SN2 reaction between the nucleoside 10 and the tosylate 11. The O6 reactivity of the ambident nucleophile hypoxanthine in 10 was a consequence of the high temperature necessary to perform the coupling reaction. Product 9 is an interesting intermediate that will be exploited for the synthesis of unprecedented O6-substituted cADPR derivatives.
Supplementary Materials
The following are available online. Figures S1–S6: copies of 1H-, 13C-NMR, COSY, HSQC, HMBC and HRESI-MS spectra of compound 9.
Author Contributions
S.D. conceived and designed the experiments; V.P. performed the synthetic experiments; M.M. performed the spectroscopic experiments; M.T. and A.M. analyzed the data; R.N. finalized the draft; S.D. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the institutional financial support to SYSBIO.ISBE.IT within the Italian Roadmap for ESFRI Research Infrastructures and by the PON-AIM RTDA_L1 (AIM 1873131-2).
Data Availability Statement
No data available.
Acknowledgments
The authors are grateful to Luisa Cuorvo for the technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Borbone, N.; Piccialli, G.; Roviello, G.N.; Oliviero, G. Nucleoside analogues and nucleoside precursors as drugs in the fight against SARS-CoV-2 and other coronaviruses. Molecules 2021, 26, 986. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.K.; Seley-Radtke, K.L. The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold. Antivir. Res. 2019, 162, 5–21. [Google Scholar] [CrossRef]
- Guinan, M.; Benckendor, C.; Smith, M.; Miller, G.J.; Way, S.; Derek, J.; Mcphee, J. Evaluation Analogues Evaluation of Anticancer Anticancer Nucleoside. Molecules 2020, 25, 2050. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol. Lett. 2019, 205, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ewald, B.; Sampath, D.; Plunkett, W. Nucleoside analogues: Molecular mechanisms signaling cell death. Oncogene 2008, 27, 6522–6537. [Google Scholar] [CrossRef] [PubMed]
- Zarrilli, F.; Amato, F.; Morgillo, C.M.C.M.; Pinto, B.; Santarpia, G.; Borbone, N.; D’Errico, S.; Catalanotti, B.; Piccialli, G.; Castaldo, G.; et al. Peptide nucleic acids as miRNA target protectors for the treatment of cystic fibrosis. Molecules 2017, 22, 1144. [Google Scholar] [CrossRef] [PubMed]
- Poppe, H.; Rybalkin, S.D.; Rehmann, H.; Hinds, T.R.; Tang, X.B.; Christensen, A.E.; Schwede, F.; Genieser, H.G.; Bos, J.L.; Doskeland, S.O.; et al. Cyclic nucleotide analogues as probes of signaling pathways. Nat. Methods 2008, 5, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.M.; Piazza, G.A.; Tinsley, H.N. The role of cyclic nucleotide signaling pathways in cancer: Targets for prevention and treatment. Cancers 2014, 6, 436–458. [Google Scholar] [CrossRef] [PubMed]
- Clapper, D.L.; Walseth, T.F.; Dargie, P.J.; Lee, H.C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 1987, 262, 9561–9568. [Google Scholar] [CrossRef]
- Guse, A.H. Calcium mobilizing second messengers derived from NAD. Biochim. Biophys. Acta 2015, 1854, 1132–1137. [Google Scholar] [CrossRef]
- Gul, R.; Park, J.H.; Kim, S.Y.; Jang, K.Y.; Chae, J.K.; Ko, J.K.; Kim, U.H. Inhibition of ADP-ribosyl cyclase attenuates angiotensin II-induced cardiac hypertrophy. Cardiovasc. Res. 2009, 81, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.V.L.; Walseth, T.F. Medicinal chemistry and pharmacology of cyclic ADP-ribose. Curr. Mol. Med. 2004, 4, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Tsuzuki, T.; Murayama, T.; Kameda, T.; Kumaki, Y.; Sakurai, T.; Fukuda, H.; Watanabe, M.; Arisawa, M.; Shuto, S. Synthesis of 8-Substituted Analogues of Cyclic ADP-4-Thioribose and Their Unexpected Identification as Ca2+-Mobilizing Full Agonists. J. Med. Chem. 2017, 60, 5868–5875. [Google Scholar] [CrossRef]
- Watt, J.M.; Graeff, R.; Thomas, M.P.; Potter, B.V.L. Second messenger analogues highlight unexpected substrate sensitivity of CD38: Total synthesis of the hybrid “l-cyclic inosine 5′-diphosphate ribose”. Sci. Rep. 2017, 7, 16100. [Google Scholar] [CrossRef]
- Sato, T.; Watanabe, M.; Tsuzuki, T.; Takano, S.; Murayama, T.; Sakurai, T.; Kameda, T.; Fukuda, H.; Arisawa, M.; Shuto, S. Design, Synthesis, and Identification of 4″α-Azidoethyl-cyclic ADP-Carbocyclic-ribose as a Highly Potent Analogue of Cyclic ADP-Ribose, a Ca2+-Mobilizing Second Messenger. J. Med. Chem. 2016, 59, 7282–7286. [Google Scholar] [CrossRef] [PubMed]
- Galeone, A.; Mayol, L.; Oliviero, G.; Piccialli, G.; Varra, M. Synthesis of a novel N-1 carbocyclic, N-9 butyl analogue of cyclic ADP ribose (cADPR). Tetrahedron 2002, 58, 363–368. [Google Scholar] [CrossRef]
- Wagner, G.K.; Guse, A.H.; Potter, B.V.L. Rapid synthetic route toward structurally modified derivatives of cyclic adenosine 5’-diphosphate ribose. J. Org. Chem. 2005, 70, 4810–4819. [Google Scholar] [CrossRef] [PubMed]
- Mahal, A.; D’Errico, S.; Borbone, N.; Pinto, B.; Secondo, A.; Costantino, V.; Tedeschi, V.; Oliviero, G.; Piccialli, V.; Piccialli, G. Synthesis of cyclic N1-pentylinosine phosphate, a new structurally reduced cADPR analogue with calcium-mobilizing activity on PC12 cells. Beilstein J. Org. Chem. 2015, 11, 2689–2695. [Google Scholar] [CrossRef]
- D’Errico, S.; Borbone, N.; Catalanotti, B.; Secondo, A.; Petrozziello, T.; Piccialli, I.; Pannaccione, A.; Costantino, V.; Mayol, L.; Piccialli, G.; et al. Synthesis and Biological Evaluation of a New Structural Simplified Analogue of cADPR, a Calcium-Mobilizing Secondary Messenger Firstly Isolated from Sea Urchin Eggs. Mar. Drugs 2018, 16, 89. [Google Scholar] [CrossRef]
- D’Errico, S.; Basso, E.; Falanga, A.P.A.P.; Marzano, M.; Pozzan, T.; Piccialli, V.; Piccialli, G.; Oliviero, G.; Borbone, N. New linear precursors of cIDPR derivatives as stable analogues of cADPR: A potent second messenger with Ca2+-Modulating activity isolated from sea urchin eggs. Mar. Drugs 2019, 17, 476. [Google Scholar] [CrossRef]
- D’Errico, S.; Greco, F.; Patrizia Falanga, A.; Tedeschi, V.; Piccialli, I.; Marzano, M.; Terracciano, M.; Secondo, A.; Roviello, G.N.; Oliviero, G.; et al. Probing the Ca2+ mobilizing properties on primary cortical neurons of a new stable cADPR mimic. Bioorg. Chem. 2021, 117, 105401. [Google Scholar] [CrossRef]
- Oliviero, G.; D’Errico, S.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Piccialli, G.; Mayol, L. A solid-phase approach to the synthesis of N-1-alkyl analogues of cyclic inosine-diphosphate-ribose (cIDPR). Tetrahedron 2010, 66, 1931–1936. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Solid-phase synthesis of a new diphosphate 5-aminoimidazole-4-carboxamide riboside (AICAR) derivative and studies toward cyclic AICAR diphosphate ribose. Molecules 2011, 16, 8110–8118. [Google Scholar] [CrossRef]
- Bonnac, L.F.; Dreis, C.D.; Geraghty, R.J. Structure activity relationship, 6-modified purine riboside analogues to activate hSTING, stimulator of interferon genes. Bioorgan. Med. Chem. Lett. 2019, 126819. [Google Scholar] [CrossRef] [PubMed]
- Hulpia, F.; Bouton, J.; Campagnaro, G.D.; Alfayez, I.A.; Mabille, D.; Maes, L.; de Koning, H.P.; Caljon, G.; Van Calenbergh, S. C6–O-alkylated 7-deazainosine nucleoside analogues: Discovery of potent and selective anti-sleeping sickness agents. Eur. J. Med. Chem. 2020, 188, 112018. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Oliviero, G.; Amato, J.; Borbone, N.; Cerullo, V.; Hemminki, A.; Piccialli, V.; Zaccaria, S.; Mayol, L.; Piccialli, G. Synthesis and biological evaluation of unprecedented ring-expanded nucleosides (RENs) containing the imidazo[4,5-d][1,2,6]oxadiazepine ring system. Chem. Commun. 2012, 48, 9310–9312. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Saleh, S.; Blair, I.A. Selective Deprotection of Silyl Ethers. Tetrahedron Lett. 1989, 30, 19–22. [Google Scholar] [CrossRef]
- Leškovskis, K.; Zaķis, J.M.; Novosjolova, I.; Turks, M. Applications of Purine Ring Opening in the Synthesis of Imidazole, Pyrimidine, and New Purine Derivatives. Eur. J. Org. Chem. 2021, 2021, 5027–5052. [Google Scholar] [CrossRef]
- Shuto, S.; Shirato, M.; Sumita, Y.; Ueno, Y.; Matsuda, A. Nucleosides and Nucleotides. 173. Synthesis of Cyclic IDP-carbocyclic-ribose, a Stable Mimic of Cyclic ADP-Ribose. Significant Facilitation of the Intramolecular Condensation Reaction of N-1-(Carbocyclic-ribosyl)inosine 5’’, 6’’-Diphosphate Derivatives by an 8-Bromo-Sustitution at the Hypoxanthine Moiety. J. Org. Chem. 1998, 63, 1986–1994. [Google Scholar]
- De Napoli, L.; Di Fabio, G.; Messere, A.; Montesarchio, D.; Piccialli, G.; Varra, M. Synthetic studies on the glycosylation of the base residues of inosine and uridine. J. Chem. Soc. Perkin Trans. 1999, 1, 3489–3493. [Google Scholar] [CrossRef]
- Hyde, R.M.; Broom, A.D.; Buckheit, R.W. Antiviral amphipathic oligo- and polyribonucleotides: Analogue development and biological studies. J. Med. Chem. 2003, 46, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Karnekanti, R.; Hanumaiah, M.; Sharma, G.V.M. Stereoselective Total Synthesis of (+)-Anamarine and 8-epi-(-)-Anamarine from D-Mannitol. Synthesis 2015, 47, 2997–3008. [Google Scholar] [CrossRef][Green Version]
- Lanier, M.L.; Park, H.; Mukherjee, P.; Timmerman, J.C.; Ribeiro, A.A.; Widenhoefer, R.A.; Hong, J. Formal Synthesis of (+)-Laurencin by Gold(I)-Catalyzed Intramolecular Dehydrative Alkoxylation. Chem.-A Eur. J. 2017, 23, 7180–7184. [Google Scholar] [CrossRef] [PubMed]
- Véliz, E.A.; Beal, P.A. 6-Bromopurine nucleosides as reagents for nucleoside analogue synthesis. J. Org. Chem. 2001, 66, 8592–8598. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, H.S.; Pauff, S.M.; Kosmidis, T.D.; Taladriz-Sender, A.; Rutherford, O.I.; Hatit, M.Z.C.; Fenner, S.; Watson, A.J.B.; Burley, G.A. Modular, Step-Efficient Palladium-Catalyzed Cross-Coupling Strategy to Access C6-Heteroaryl 2-Aminopurine Ribonucleosides. Org. Lett. 2017, 19, 3759–3762. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.J.; Shurtleff, V.W.; Johnson, A.M.; El Marrouni, A. Synthesis of C6-Substituted Purine Nucleoside Analogues via Late-Stage Photoredox/Nickel Dual Catalytic Cross-Coupling. ACS Med. Chem. Lett. 2021, 12, 662–666. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
