Abstract
SN2 rection between 4-(tert-butyldimethylsilyl)hex-5-yn-1-yl 4-methylbenzenesulfonate and NaN3 in DMF at 80 °C provided (6-azidohex-1-yn-3-yl)(tert-butyl)dimethylsilane intermediate, which underwent in situ intramolecular thermal Huisgen azide–alkyne cycloaddition reaction. This one-pot process gave 4-(tert-butyldimethylsilyl)-4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine in 78% yield.
1. Introduction
The term triazolopyridine includes five types of heterocyclic systems with one subtype being [1,2,3]triazolo[1,5-a]pyridine. The synthesis and applications of the fully aromatic congeners have been reviewed in 2002 [1] and 2010 [2]. Their applications range from fluorescent materials to building blocks in supramolecular chemistry, which are known to form polynuclear complexes with different metal ions. However, they are less studied in medicinal chemistry, although some examples include Ca2+ channel inhibitors, blockers of α1-adrenoreceptors and neural nitric oxide synthase inhibitors [2].
On the other hand, partially saturated [1,2,3]triazolo[1,5-a]pyridine moiety has been included as side chain in novel potassium channel modulators for the treatment and prevention of disorders of the nervous system [3] (Figure 1). A similar substituent has also been researched during the elaboration of selective cyclin dependent kinase-9 inhibitor, which was developed for the treatment of hematological malignancies [4]. Recently, the structural core of 4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine has found application in the construction of bicyclic fused triazolium ionic liquids, which were designed for the chemoselective extraction of copper(II) ions and also histidine-containing peptides [5]. Other application fields of these low viscosity ionic liquids are dye-sensitized solar cells, in which the bicyclic 1,2,3-triazolium derivatives serve as nonvolatile electrolytes. It has been mentioned that 4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine-derived ionic liquids outperform the more traditional imidazolium congeners [6].
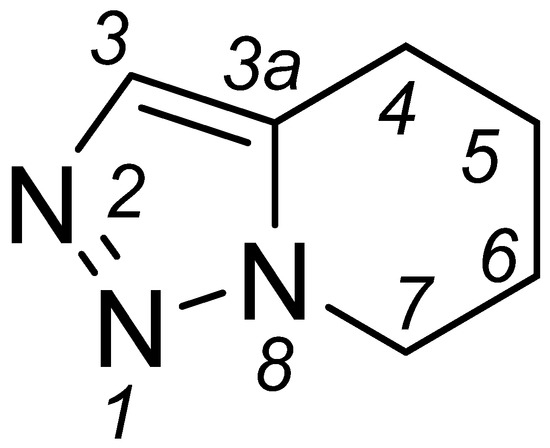
Figure 1.
Structure and atom numbering of 4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine core.
From the synthetic chemistry point of view, the aromatic [1,2,3]triazolo[1,5-a]pyridines are known to expel molecular nitrogen in the presence of transition metal catalysts and form carbene metal complexes, which are valuable synthetic intermediates in the synthesis of various heterocyclic systems [7]. The chemistry of partially hydrogenated [1,2,3]triazolo[1,5-a]pyridines is far less explored. There are only few reports on their synthesis. Thus, partially saturated systems can be prepared by hydrogenation of the pyridine part of the fused triazolopyridine system, as has been described in the first reports of synthesis of 4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridines containing substituents in the triazole part [8,9]. The Yus group has reported a one-pot SN2 reaction—the dipolar cycloaddition reaction sequence of 6-chlorohex-1-yne and NaN3 in the presence of the copper nanoparticles on activated carbon. This provided 4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine in a copper(I)-catalyzed azide–alkyne dipolar cycloaddition reaction [10,11]. The intramolecular cyclization of 6-azidohex-1-yne was also studied in the presence of sulfanyl radicals and unsubstituted 4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine was obtained in 45% yield [12]. In another study, the latter partially saturated bicycle was obtained unexpectedly from N-acylated 6-amino-1-diazohexan-2-one by N-deprotection-induced imine formation followed by tautomerization into the fused triazole [13]. It was shown that 6-azidohex-1-yne intermediate, which in another report was obtained from the corresponding mesylate, can also be cyclized in the thermal Huisgen cycloaddition reaction in the absence of copper(I) catalyst [5].
2. Results and Discussion
In our efforts to explore 1,2-silyl group shift in propargyl silanes [14,15,16], we envisaged the synthesis of (6-azidohex-1-yn-3-yl)(tert-butyl)dimethylsilane 3 (Scheme 1). During the SN2 process of tosylate–azide exchange the latter in situ underwent thermal Huisgen cycloaddition in the absence of the copper(I) catalyst, similar to that reported by the Chu group [5]. Thus, the first silyl-functionalized 4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine (4) was obtained. The strategic starting material 1 was obtained by retro-Brook rearrangement of O-silylated hex-5-yn-1-ol as described before [17]. Next, O-tosylation provided intermediate 2. It is important to note, that compound 2 appeared to be a rather unstable molecule, for which the standard chromatographic purification on silica gel is not advisable. Instead, the reaction 1→2 should be conducted to the maximum conversion and the product should be isolated only by extractive methods and directly employed in the next transformation. The obtained product 4 and its possible congeners with differently substituted silyl group can be further applied in various reactions known for silane chemistry. Among others, these include Hiyama couplings [18] and Fleming–Tamao oxidations [19,20].
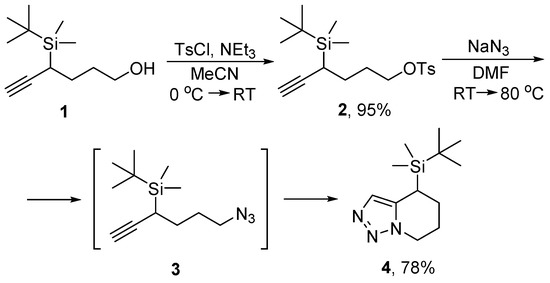
Scheme 1.
Synthesis of 4-(tert-butyldimethylsilyl)-4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine 4.
The molecular structure of compound 4 was unambiguously established by spectroscopic methods. The analysis of the spin coupling constants and 2D NOESY spectrum revealed that the TBS-group is situated in pseudo-equatorial position (Figure 2). It is possible to state that such a conformational anchor can stabilize the overall conformation of compound 4. The best constant analysis was possible for the 1H NMR spectrum in deutero benzene. It should be mentioned that coupling constants between H-C(5) and H-C(6) were not attributed due to complex signal shape in all tested solvents (CDCl3, C6D6, MeOD-d4, DMSO-d6, THF-d8) and at all tested temperatures (25→80 °C). On the other hand, the chemical shifts of compound 4 in its 1H and 13C NMR were compared to those previously reported for unsubstituted 4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine core (Table 1) [5,13]. They were practically identical with exception of H-C(4), which was shifted upfield by 0.38 ppm due to the attached TBS-group.
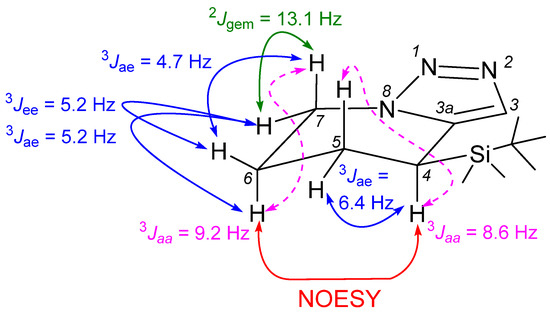
Figure 2.
Observed coupling constants (1H NMR, C6D6) and NOESY effects of compound 4.

Table 1.
Characteristic NMR shifts of the fused core of product 4 and their comparison with published data [5,13] for unsubstituted 4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine.
Also the FTIR analysis of compound 4 showed the expected absorption bands in the regions that were previously reported for similar structures [8,21]: 2958, 2927, 1527, 1470, 1248, 1113, 1065, 993 cm−1. The absorption bands at 1470, 1113, 1065 and 993 cm−1 are, in general, considered characteristic for 1,2,3-triazoles [21].
3. Materials and Methods
Reaction solvents (MeCN, DMF) were dried using standard drying agents and distilled prior to use. Commercially available reagents were used as received. All reactions were followed by TLC on E. Merck Kieselgel 60 F254 and visualized by using UV lamp or developed using generic KMnO4 stain. Column chromatography was performed on silica gel (60 Å, 40–63 μm, Upasil®). 1H and 13C-NMR spectra were recorded using a Bruker Avance 500 MHz spectrometer. Chemical shifts (δ) are reported in ppm and coupling constants (J) in Hz. Residual solvent or solvent peaks were used as internal reference (CDCl3, δ 7.26 ppm for 1H-NMR; CDCl3, δ 77.16 ppm for 13C-NMR). Multiplicities are indicated as follows: s (singlet), d (doublet), t (triplet), m (multiplet). Compound purity assessments were performed by quantitative NMR, where 1,1′-methylenedibenzene was used as internal standard. IR spectra were recorded as thin films on an FT-IR Perkin-Elmer Spectrum 100 spectrometer (4000–450 cm−1). High-resolution mass spectra (ESI) were recorded with an Agilent 1290 Infinity series UPLC connected to an Agilent 6230 TOF mass spectrometer (calibration at m/z 121.050873 and m/z 922.009798). The starting material 1 was prepared from tert-butyl(hex-5-yn-1-yloxy)dimethylsilane according to the literature procedure [17].
4-(tert-Butyldimethylsilyl)hex-5-yn-1-yl 4-methylbenzenesulfonate2. Triethylamine (27 mL, 0.19 mol, 5 eq.) was added to a solution of 4-(tert-butyldimethylsilyl)hex-5-yn-1-ol (1) (7.889 g, 0.037 mmol, 1.0 eq.) in MeCN (50 mL) at 0 °C followed by 4-toluenesulfonyl chloride (8.970 g, 0.05 mol, 1.3 eq.). The resulting reaction mixture was stirred for 1 h at 0 °C. The solution gradually obtained an orange color and formation of precipitate was observed. The resulting mixture was stirred for another 2 h at room temperature. The solvent was removed under reduced pressure. The obtained residue was dissolved in DCM (50 mL) and washed with a saturated aqueous solution of NH4Cl (3 × 50 mL), brine (50 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was dissolved in hexanes (10–15 mL) and filtered through a thin layer of silica gel. The filtrate was concentrated to obtain product 2 as a yellow-colored oil (14.06 g, 92% 1H NMR purity; 95% yield) by NMR. Product 2 can be further purified by silica gel column chromatography (45→100% DCM/hexanes) to obtain a colorless oil, but the product undergoes partial degradation under such conditions. Rf = 0.63 (DCM). 1H-NMR (500 MHz, CDCl3): δ 7.79 (d, 3JH-H = 8.3 Hz, 2H, H-C(2′′)), 7.34 (d, 3JH-H = 8.3 Hz, 2H, H-C(3′′)), 4.17–4.00 (m, 2H, H2C(1)), 2.45 (s, 3H, H3C(5′′), 2.09–1.98 (m, 1H, HaC(2)), 1.95 (d, 4JH-H = 2.8 Hz, 1H, HC(6)), 1.79–1.68 (m, 1H, HbC(2)), 1.66 (dt, 3JH-H = 11.9 Hz, 4JH-H = 2.8 Hz, 1H, HC(4)), 1.59–1.48 (m, 1H, HaC(3)), 1.41–1.32 (m, 1H, HbC(3)), 0.93 (s, 9H, H3C(1′)), 0.05 (s, 3H, H3C(2′)), −0.02 (s, 3H, H3C(2′)). 13C-NMR (126 MHz, CDCl3): δ 144.8, 133.4, 130.0, 128.1, 86.4, 70.3, 70.0, 28.5, 27.2, 25.7, 21.8, 17.7, 16.2, −7.1, −7.3. IR (FTIR): 3312, 2956, 2930, 2897, 2858, 2175, 2099, 1926, 1716, 1641, 1599, 1496, 1471, 1361, 1807, 1292, 1251, 1189, 1120, 1098, 1070 cm−1. HRMS (ESI): m/z calculated for [C19H30O3SSi + H]+ 367.1758, found 367.1728. These spectra data can be downloaded in Supplementary Materials.
4-(tert-Butyldimethylsilyl)-4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine 4. The 4-(tert-Butyldimethylsilyl)hex-5-yn-1-yl 4-methylbenzenesulfonate (2) (155 mg, 0.42 mmol, 1.0 eq.) was added to a stirred solution of sodium azide (45 mg, 0.69 mmol, 1.6 eq.) in anhydrous DMF (3 mL) under inert argon atmosphere. The resulting reaction mixture was stirred for 20 min. at room temperature. The yellow-colored solution was then heated at 80 °C for 66 h. A saturated aqueous solution of NaHCO3 was added to the reaction mixture at room temperature. The product was then extracted with toluene (3 × 10 mL). The organic phases were collected separately and concentrated under reduced pressure. The obtained residue was dissolved in chloroform (10 mL) and washed with water (2 × 10 mL), brine (10 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (20→40% EtOAc/hexanes) and product 4 (78 mg, 78%) was obtained as a colorless oil. Rf = 0.20 (40% EtOAc/Hex). 1H-NMR (500 MHz, CDCl3): δ 7.37 (s, 1H, H-C(3)), 4.52–4.44 (m, 1H, HaC(7)), 4.28–4.19 (m, 1H, HbC(7)), 2.46 (dd, 3JH-H = 8.6, 6.4 Hz, 1H, HC(4)), 2.15–2.06 (m, 2H, H2C(5,6)), 1.95–1.82 (m, 2H, H2C(5,6)), 0.95 (s, 9H, H3C(1′)), 0.05 (s, 3H, H3C(2′)), 0.03 (s, 3H, H3C(2′)). 1H NMR (500 MHz, C6D6) δ 7.44 (s, 1H, H-C(3)), 3.96 (dt, 2JH-H = 13.1 Hz, 3JH-H = 5.2 Hz, 1H, HaC(7)), 3.62 (ddd, 2JH-H = 13.1 Hz, 3JH-H = 9.2, 4.7 Hz, 1H, HbC(7)), 1.91 (dd, 3JH-H = 8.6, 6.4 Hz, 1H, HC(4)), 1.42–1.32 (m, 1H, HaC(5)), 1.29–1.14 (m, 2H, HbC(5), HaC(6)), 1.07–0.95 (m, 1H, HbC(6)), 0.75 (s, 9H, H3C(1′)), −0.18 (s, 3H, H3C(2′)), −0.24 (s, 3H, H3C(2′)). 13C-NMR (126 MHz, CDCl3): δ 135.8, 130.5, 46.2, 27.4, 23.4, 23.1, 19.3, 17.7, −5.3, −6.3. IR (FTIR): 2958, 2927, 2882, 2856, 1527, 1470, 1451, 1431, 1364, 1248, 1232, 1158, 1113, 1065, 1047, 993 cm−1. HRMS (ESI): m/z calculated for [C12H23N3Si + H]+ 238.1734, found 238.1746. These spectra data can be downloaded in Supplementary Materials.
4. Conclusions
The 4-(tert-butyldimethylsilyl)-4,5,6,7-tetrahydro-[1,2,3]triazolo[1,5-a]pyridine can be obtained with 78% yield in a one-pot process by heating a mixture of 4-(tert-butyldimethylsilyl)hex-5-yn-1-yl 4-methylbenzenesulfonate and sodium azide. Its structural analysis by 1H NMR revealed that the bulky tert-butyldimethylsilyl group is placed in the pseudo-equatorial position.
Supplementary Materials
1H-NMR, 13C-NMR, IR spectra and HRMS (ESI) data can be downloaded.
Author Contributions
R.K. conducted synthetic experiments and prepared the manuscript; R.B. conducted NMR experiments; M.T. brought the idea, managed the research and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the Riga Technical University Grant for Master Students (R.K.) ZM-2021/19.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compound 4 are available from the authors.
References
- Jonesa, G.; Abarca, B. The Chemistry of the [1,2,3]Triazolo[1,5-a]pyridines: An Update. Adv. Heterocycl. Chem. 2010, 100, 195–252. [Google Scholar] [CrossRef]
- Abarca-González, B. The Chemistry of [1,2,3]Triazolo[1,5-a]pyridines. J. Enz. Inhib. Med. Chem. 2002, 17, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, B.L.; Gustafsson, M.; Hougaard, C.; Jacobsen, T.A.; Jefson, M.R.; Klein, J.; Larsen, J.S.; Lowe, J.A., III; McCall, J.M.; Strooebaek, D.; et al. Preparation of Cycloalkylamino Nitrogen Heterocycles as Potassium Channel Modulators for the Treatment and Prevention of Disorders. U.S. Patent US10774064B2, 15 September 2020. [Google Scholar]
- Barlaam, B.; Casella, R.; Cidado, J.; Cook, C.; De Savi, C.; Dishington, A.; Donald, C.S.; Drew, L.; Ferguson, A.D.; Ferguson, D.; et al. Discovery of AZD4573, a Potent and Selective Inhibitor of CDK9 That Enables Short Duration of Target Engagement for the Treatment of Hematological Malignancies. J. Med. Chem. 2020, 63, 15564–15590. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Chen, C.-Y.; Cheng, H.-T.; Chu, Y.-H. Exploiting 1,2,3-Triazolium Ionic Liquids for Synthesis of Tryptanthrin and Chemoselective Extraction of Copper(II) Ions and Histidine-Containing Peptides. Molecules 2016, 21, 1355. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.P.S.; Tsao, H.N.; Zakeeruddin, S.M.; Gratzel, M.; Dyson, P.J. Highly Stable Dye-Sensitized Solar Cells Based on Novel 1,2,3-Triazolium Ionic Liquids. ACS Appl. Mater. Interfaces 2014, 6, 13571–13577. [Google Scholar] [CrossRef] [PubMed]
- Yadagiri, D.; Rivas, M.; Gevorgyan, V. Denitrogenative Transformations of Pyridotriazoles and Related Compounds: Synthesis of N-Containing Heterocyclic Compounds and Beyond. J. Org. Chem. 2020, 85, 11030–11046. [Google Scholar] [CrossRef] [PubMed]
- Abarca, B.; Ballesteros, R.; Elmasnouy, M. Triazolopyridines 20. Hydrogenation reactions. Tetrahedron 1999, 55, 12881–12884. [Google Scholar] [CrossRef]
- Abarca, B.; Adam, R.; Alom, S.; Ballesteros, R.; Lopez-Molina, S. Triazolopyridines. Part 30. Hydrogen transfer reactions; pyridylcarbene formation. ARKIVOC Arch. Org. Chem. 2013, 2014, 175–186. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Click chemistry from organic halides, diazonium salts and anilines in water catalysed by copper nanoparticles on activated carbon. Org. Biomol. Chem. 2011, 9, 6385–6395. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Multicomponent Synthesis of 1,2,3-Triazoles in Water Catalyzed by Copper Nanoparticles on Activated Carbon. Adv. Synth. Catal. 2010, 352, 3208–3214. [Google Scholar] [CrossRef]
- Montevecchi, P.C.; Navacchia, M.L.; Spagnolo, P. A study of vinyl radical cyclization onto the azido group by addition of sulfanyl, stannyl, and silyl radicals to alkynyl azides. Eur. J. Org. Chem. 1998, 1998, 1219–1226. [Google Scholar] [CrossRef]
- Clark, J.S.; Hodgson, P.B.; Goldsmith, M.D.; Street, L.J. Rearrangement of ammonium ylides produced by intramolecular reaction of catalytically generated metal carbenoids. Part 1. Synthesis of cyclic amines. J. Chem. Soc. Perkin Trans. 1 2001, 24, 3312–3324. [Google Scholar] [CrossRef]
- Puriņš, M.; Mishnev, A.; Turks, M. Brønsted Acid Catalyzed 1,2-Silyl Shift in Propargyl Silanes: Synthesis of Silyl Dienes and Silyl Indenes. J. Org. Chem. 2019, 84, 3595–3611. [Google Scholar] [CrossRef] [PubMed]
- Beļaunieks, R.; Puriņš, M.; Kumpiņš, V.; Turks, M. Synthesis of 3-Silylated 3-Sulfolenes from Propargylsilanes and their Reductive Desulfitation. Chem. Heterocycl. Comp. 2021, 57, 20–25. [Google Scholar] [CrossRef]
- Beļaunieks, R.; Puriņš, M.; Turks, M. Manifestation of the β-Silicon Effect in the Reactions of Unsaturated Systems Involving a 1,2-Silyl Shift. Synthesis 2020, 52, 2147–2161. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Q.; Buevich, A.V.; Yasuda, N.; Zhang, Y.; Yang, R.; Zhang, L.K.; Martin, G.E.; Williamson, R.T. Unexpected Propargylic Retro-Brook Rearrangements in Alkynes. J. Org. Chem. 2019, 84, 10024–10031. [Google Scholar] [CrossRef] [PubMed]
- Sore, H.F.; Galloway, W.R.J.D.; Spring, D.R. Palladium-catalysed cross-coupling of organosilicon reagents. Chem. Soc. Rev. 2012, 41, 1845–1866. [Google Scholar] [CrossRef] [PubMed]
- Tamao, K. Discovery and synthetic applications of novel silicon-carbon bond cleavage reactions based on the coordination number change of organosilicon compounds. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2008, 84, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.R.; Landais, Y. The Oxidation of the Carbon-Silicon Bond. Tetrahedron 1996, 52, 7599–7662. [Google Scholar] [CrossRef]
- Tanaka, S.; Velen, S.R.; Miller, S.I. Syntheses and properties of H-1,2,3-triazoles. Tetrahedron 1973, 29, 3271–3283. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).