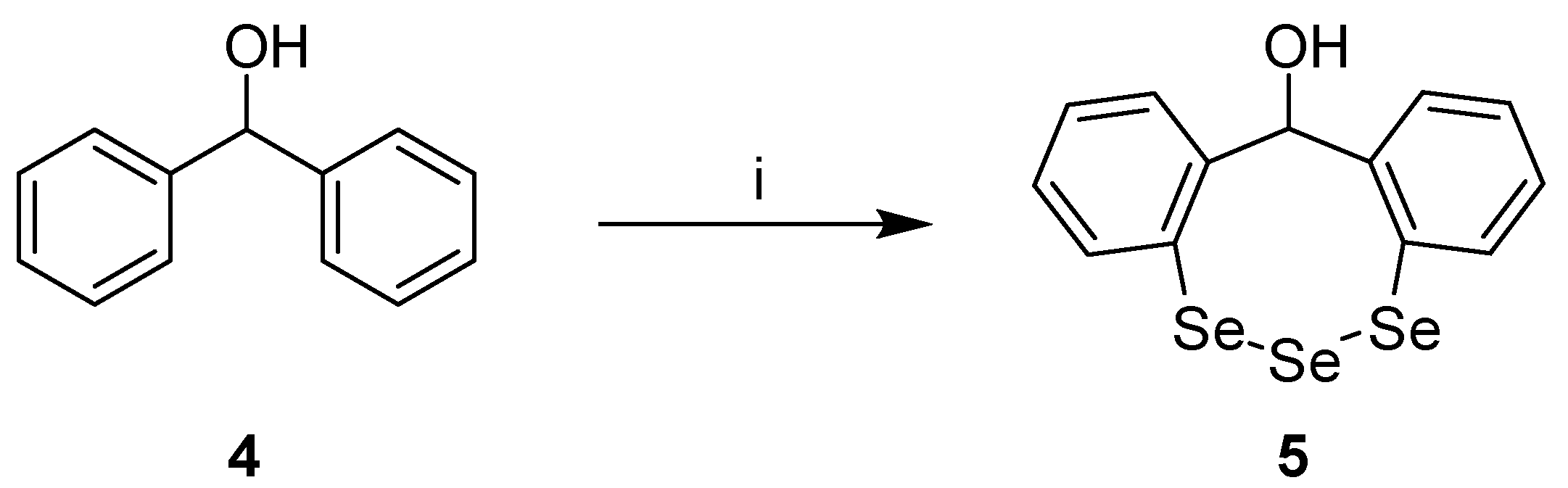
12H-Dibenzo[d,g][1,2,3]triselenocin-12-ol
Abstract
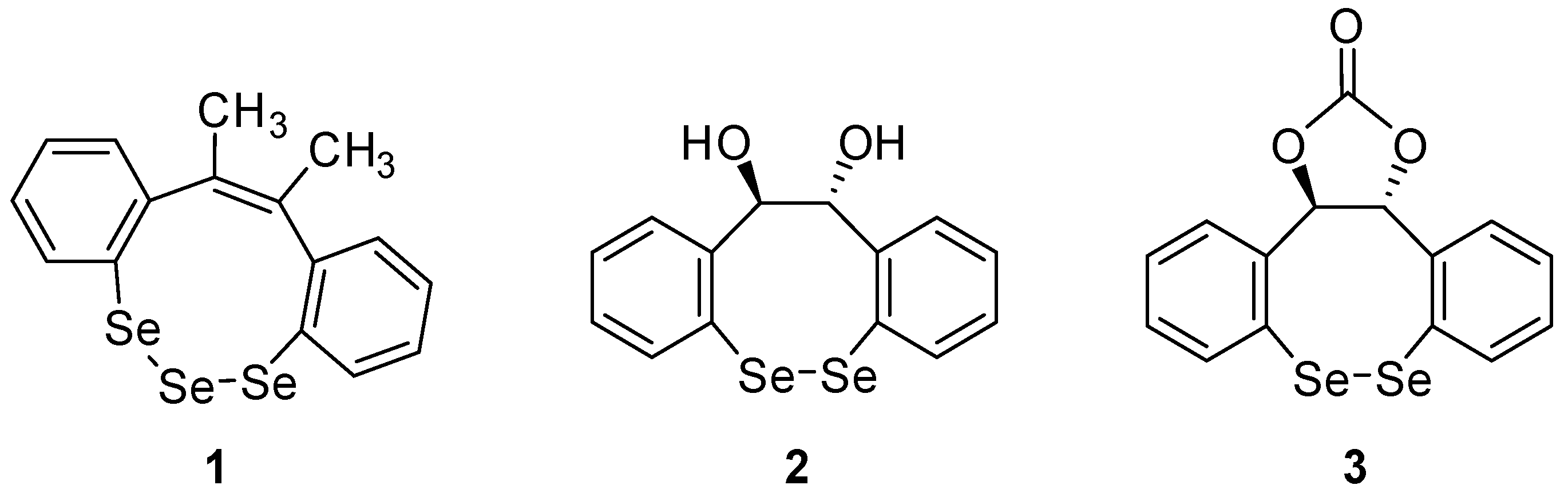
:1. Introduction
2. Results and Discussion
2.1. Chemistry
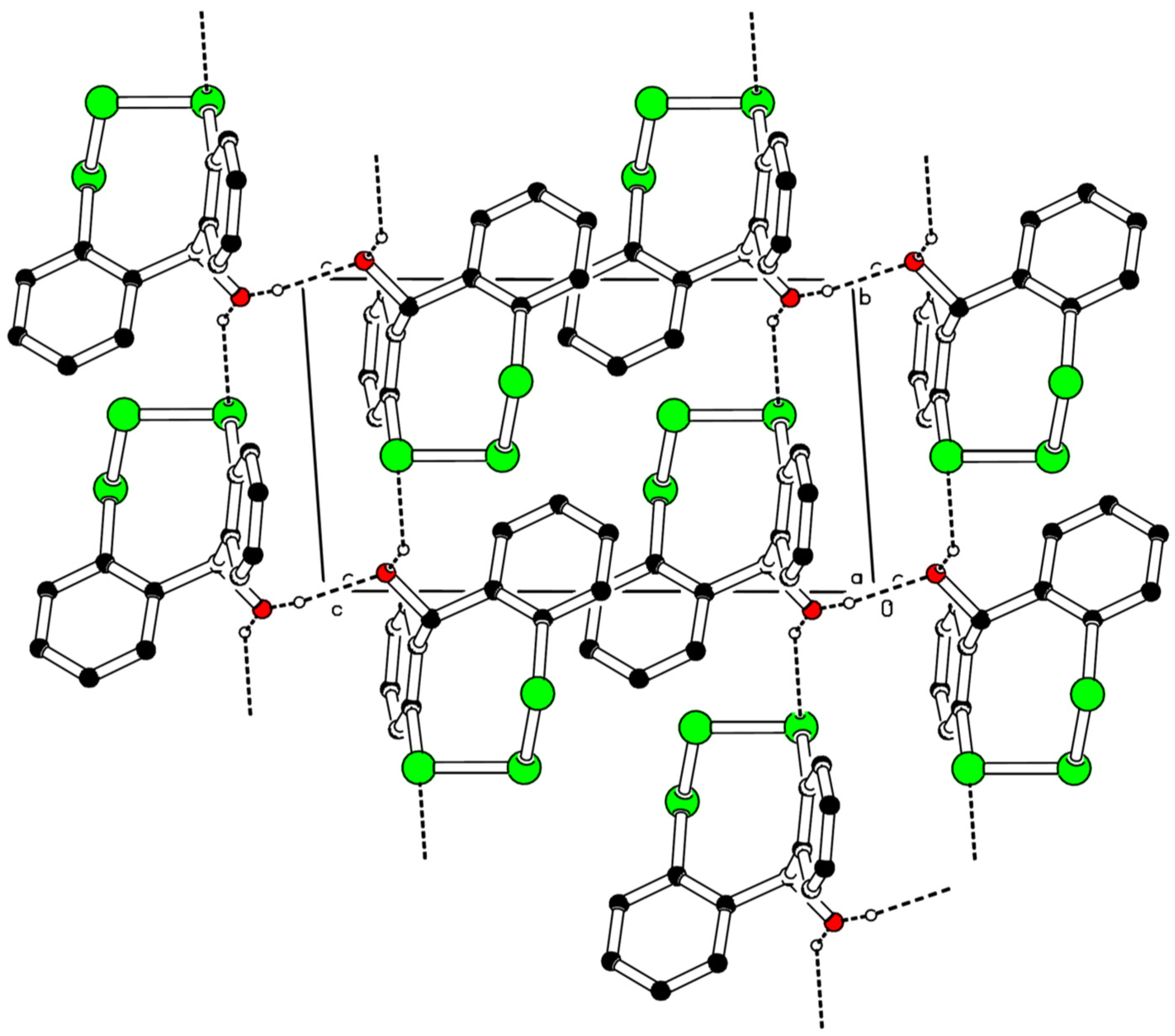
2.2. X-ray Structure
3. Materials and Methods
3.1. General
3.2. Chemistry
Synthesis of 12H-Dibenzo[d,g][1,2,3]triselenocin-12-ol (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yamazaki, S.; Yoshimura, T.; Yamabe, S.; Arai, T.; Tamura, H. Synthesis and unusual selenium extrusion reaction of a cyclic triselenide. J. Org. Chem. 1990, 55, 263–269. [Google Scholar] [CrossRef]
- Krätzschmar, F.; Ortgies, S.; Willing, R.Y.N.; Breder, A. Rational design of chiral selenium-π-acid catalysts. Catalysts 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boskovic, M.; Andreev, S.; Schollmeyer, D.; Koch, P. 12H-Dibenzo[d,g][1,2,3]triselenocin-12-ol. Molbank 2022, 2022, M1418. https://doi.org/10.3390/M1418
Boskovic M, Andreev S, Schollmeyer D, Koch P. 12H-Dibenzo[d,g][1,2,3]triselenocin-12-ol. Molbank. 2022; 2022(3):M1418. https://doi.org/10.3390/M1418
Chicago/Turabian StyleBoskovic, Marko, Stanislav Andreev, Dieter Schollmeyer, and Pierre Koch. 2022. "12H-Dibenzo[d,g][1,2,3]triselenocin-12-ol" Molbank 2022, no. 3: M1418. https://doi.org/10.3390/M1418
APA StyleBoskovic, M., Andreev, S., Schollmeyer, D., & Koch, P. (2022). 12H-Dibenzo[d,g][1,2,3]triselenocin-12-ol. Molbank, 2022(3), M1418. https://doi.org/10.3390/M1418