[4-(3,4-Dimethoxyphenyl)-3,6-dimethyl-2-phenyl-3,4-dihydroquinolin-1(2H)-yl)](furan-2-yl)methanone
Abstract
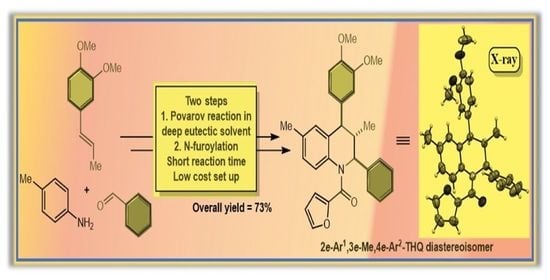
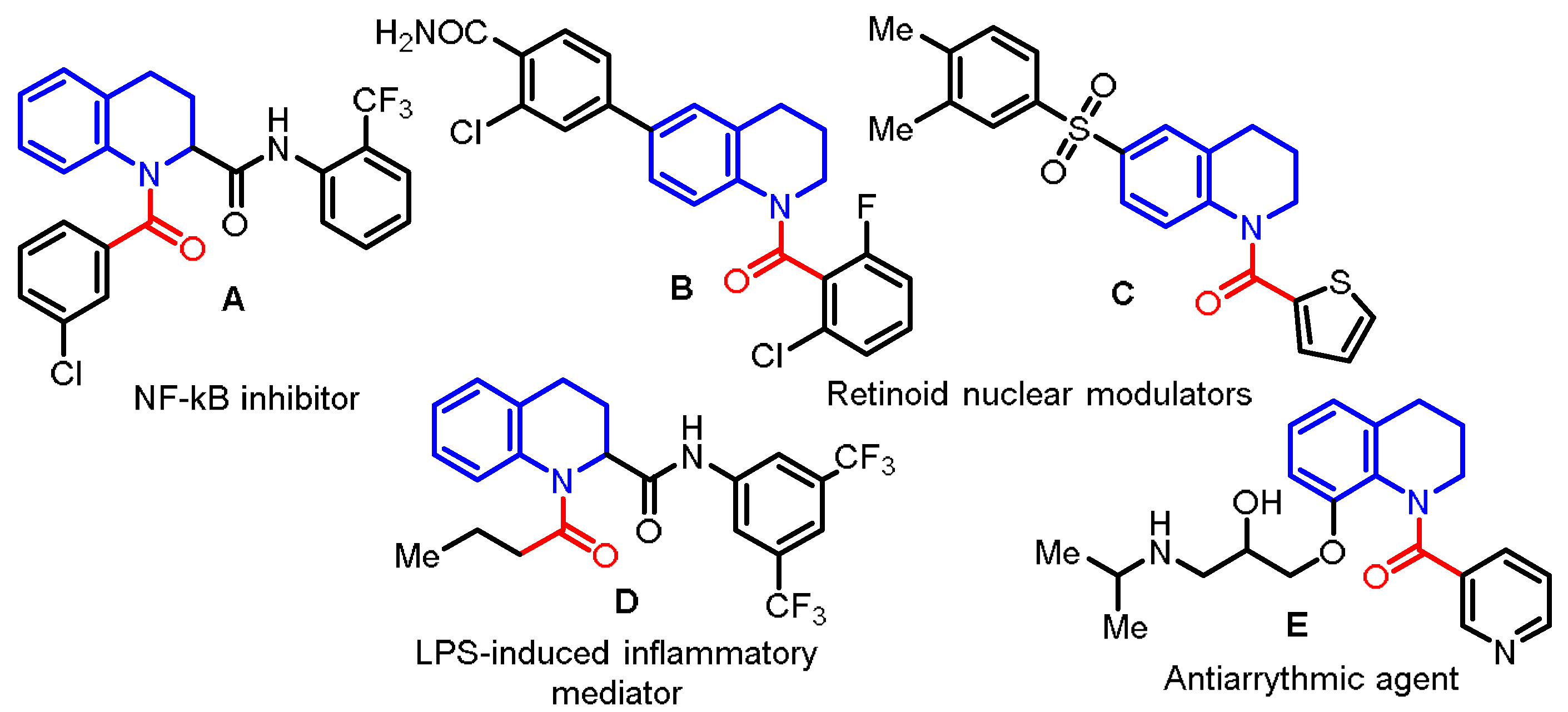
:1. Introduction
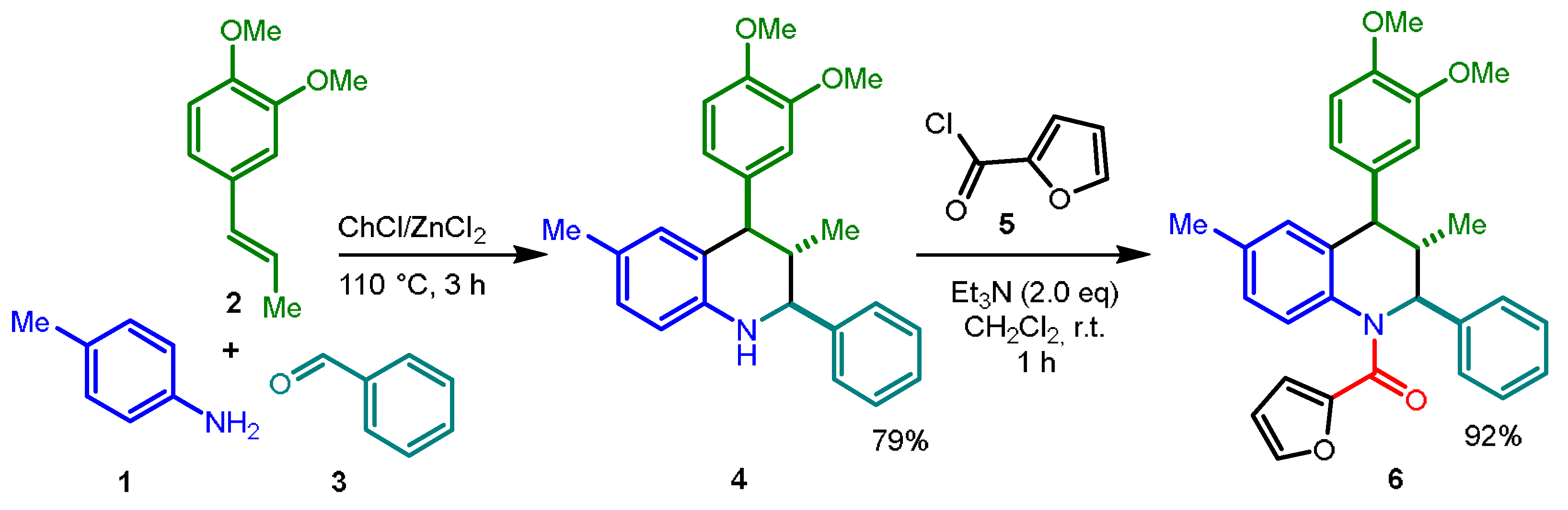
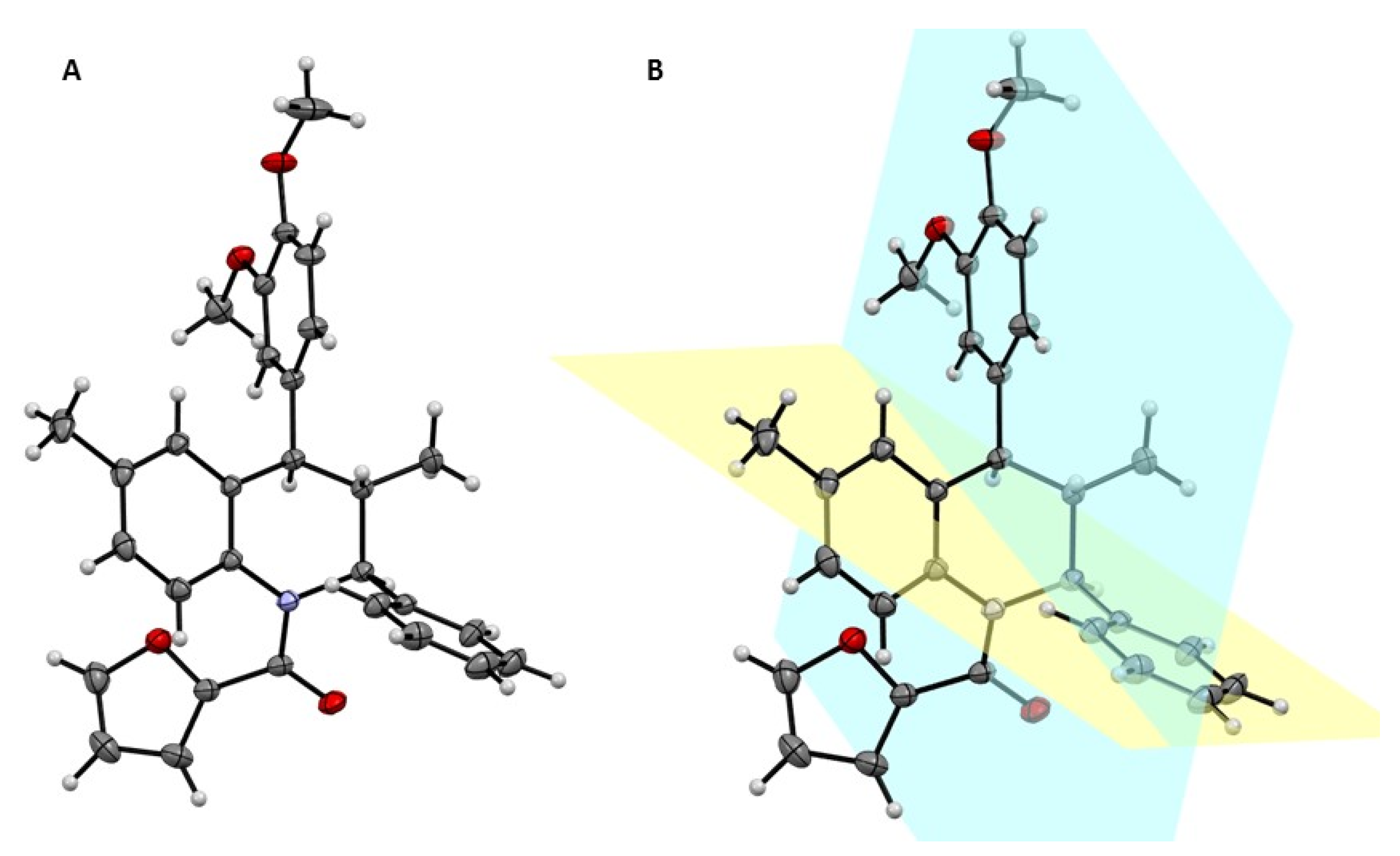
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Analysis
3.2. Synthesis of [4-(3,4-Dimethoxyphenyl)-3,6-dimethyl-2-phenyl-3,4-dihydroquinolin-1(2H)-yl)](furan-2-yl)methanone (6)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Asolkar, R.; Schröder, D.; Heckmann, R.; Lang, S.; Wagner-Döbler, I.; Laatsch, H. Helquinoline, a new tetrahydroquinoline antibiotic from Janibacter limosus. J. Antibiot. 2004, 57, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Jacquemond-Collet, I.; Hannedouche, S.; Fabre, N.; Fourasté, I.; Moulis, C. Two tetrahydroquinoline alkaloids from Galipea officinalis. Phytochemistry 1999, 51, 1167–1169. [Google Scholar] [CrossRef]
- Wright, W.; Addae-Kyereme, J.; Breen, A.G.; Brown, J.; Cox, F.; Croft, L.; Gökçek, Y.; Kendrick, H.; Phillips, M.; Pollet, L. Synthesis and evaluation of cryptolepine analogues for their potential as new antimalarial agents. J. Med. Chem. 2001, 44, 3187–3190. [Google Scholar] [CrossRef]
- Nakagawa, A.; Iwai, Y.; Hashimoto, H.; Miyazaki, N.; Oiwa, R.; Takahashi, Y.; Hirano, A.; Shibukawa, N.; Kojima, Y.; Omura, S. Virantmycin, a new antiviral antibiotic produced by a strain of Streptomyces. J. Antibiot. 1981, 34, 1408–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witherup, M.; Ransom, W.; Graham, C.; Bernard, M.; Salvatore, J.; Lumma, C.; Anderson, S.; Pitzenberger, S.M.; Varga, S.L. Martinelline and Martinellic acid, novel G-protein linked receptor antagonists from the tropical plant Martinella iquitosensis (Bignoniaceae). J. Am. Chem. Soc. 1995, 117, 6682–6685. [Google Scholar] [CrossRef]
- Vargas Méndez, L.Y.; Zacchino, S.; Kouznetsov, V.V. Synthesis of new 4-methyl-2-(4-pyridyl)-1,2,3,4 tetrahydroquinolines as potent antifungal compounds. J. Braz. Chem. Soc. 2010, 21, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Bedoya, M.; Abad, M.; Calonge, E.; Astudillo, L.; Gutierrez, M.; Kouznetsov, V.V.; Alcami, J.; Bermejo, P. Quinoline-based compounds as modulators of HIV transcription through NF-κB and Sp1 inhibition. Antivir. Res. 2010, 87, 338–344. [Google Scholar] [CrossRef]
- Su, D.S.; Lim, J.J.; Tinney, E.; Wan, B.L.; Young, M.B.; Anderson, K.D.; Rudd, D.; Munshi, V.; Bahnck, C.; Felock, P.J.; et al. Substituted tetrahydroquinolines as potent allosteric inhibitors of reverse transcriptase and its key mutants. Bioorg. Med. Chem. Lett. 2009, 19, 5119–5123. [Google Scholar] [CrossRef]
- Muñoz, A.; Sojo, F.; Merchan, D.R.; Kouznetsov, V.V.; Arvelo, F. Cytotoxic effects of new trans-2,4-diaryl-3-methyl-1,2,3,4-tetrahydroquinolines and their interaction with antitumoral drugs gemcitabine and paclitaxel on cellular lines of human breast cancer. Chem.-Biol. Interact. 2011, 189, 215–221. [Google Scholar] [CrossRef]
- Jo, H.; Choi, M.; Kumar, A.S.; Jung, Y.; Kim, S.; Yun, J.; Lee, H. Development of novel 1,2,3,4-tetrahydroquinoline scaffolds as potent NF-κB inhibitors and cytotoxic agents. ACS Med. Chem. Lett. 2016, 7, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Bui, B.P.; Oh, Y.; Lee, H.; Cho, J. Inhibition of inflammatory mediators and cell migration by 1,2,3,4-tetrahydroquinoline derivatives in LPS-stimulated BV2 microglial cells via suppression of NF-κB and JNK pathway. Int. Immunopharmacol. 2020, 80, 106231. [Google Scholar] [CrossRef] [PubMed]
- Enyedy, I.J.; Powell, N.A.; Caravella, J.; van Vloten, K.; Chao, J.; Banerjee, D.; Marcotte, D.; Silvian, L.; McKenzie, A.; Hong, V.S. Discovery of biaryls as RORγ inverse agonists by using structure-based design. Bioorg. Med. Chem. Lett. 2016, 26, 2459–2463. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, S.; Kimura, T.; Arita, M. Nicainoprol. Cardiovasc. Drug Rev. 1991, 9, 223–236. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Nandi, S.; Adhikari, P.; Sarmah, B.K.; Konwar, M.; Das, A. Boric acid catalyzed chemoselective reduction of quinolines. Org. Biomol. Chem. 2020, 18, 1214–1220. [Google Scholar] [CrossRef]
- Al-Qirim, T.; Shattat, G.; Sheikha, G.A.; Sweidan, K.; Al-Hiari, Y.; Jarab, A. Synthesis of novel N-(4-benzoylphenyl)-2-furamide derivatives and their pharmacological evaluation as potent antihyperlipidemic agents in rats. Drug Res. 2015, 65, 158–163. [Google Scholar] [CrossRef]
- Farrukh, U.B.; Bilal, A.; Zahid, H.; Iqbal, M.; Manzoor, S.; Firdous, F.; Furqan, M.; Azeem, M.; Emwas, A.-H.; Alazmi, M.; et al. Synthesis and Evaluation of Novel Carboxamides Capable of Causing Centrosome Declustering and Apoptosis in Breast Cancer Cells. ChemistrySelect 2022, 7, e202104218. [Google Scholar] [CrossRef]
- Sweidan, K.; Idrees, G.; Abu-Qatouseh, L.; Tahir, M.N.; Khanfar, M.; Joshi, R.; Eyad, M.; Mubarak, M.S. Synthesis, Characterization, and Antimicrobial Evaluation of New Furan-2-Carboxamide Derivatives. Lett. Org. Chem. 2022, 19, 314–325. [Google Scholar] [CrossRef]
- Matiichuk, Y.; Gorak, Y.; Martyak, R.; Chaban, T.; Ogurtsov, V.; Chaban, I.; Matiychuk, V. Synthesis and antimicrobial activity of 4-(5-ARYL-2-FUROYL) morpholines and 4-[(5-ARYL-2-FURYL) carbonothioyl] morpholines. Pharmacia 2021, 68, 175–179. [Google Scholar] [CrossRef]
- Babu, T.H.; Shanthi, G.; Perumal, P.T. A facile one-pot synthesis of N-substituted tetrahydroquinolines. Tetrahedron Lett. 2009, 50, 2881–2884. [Google Scholar] [CrossRef]
- Adhikari, P.; Bhattacharyya, D.; Nandi, S.; Kancharla, P.K.; Das, A. Reductive Alkylation of Quinolines to N-Alkyl Tetrahydroquinolines Catalyzed by Arylboronic Acid. Org. Lett. 2021, 23, 2437–2442. [Google Scholar] [CrossRef]
- Muthukrishnan, I.; Sridharan, V.; Menéndez, J.C. Progress in the Chemistry of Tetrahydroquinolines. Chem. Rev. 2019, 119, 5057–5191. [Google Scholar] [CrossRef] [PubMed]
- Glushkov, V.A.; Tolstikov, A.G. Synthesis of substituted 1,2,3,4-tetrahydroquinones by the Povarov reaction. New potentials of the classical reaction. Russ. Chem. Rev. 2008, 77, 137–159. [Google Scholar] [CrossRef]
- Kouznetsov, V.V. Recent synthetic developments in a powerful imino Diels-Alder reaction (Povarov reaction): Application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 2009, 65, 2721–2750. [Google Scholar] [CrossRef]
- Sridharan, V.; Suryavanshi, P.A.; Menéndez, C. Advances in the chemistry of tetrahydroquinolines. Chem. Rev. 2011, 111, 7157–7259. [Google Scholar] [CrossRef]
- Forero, J.S.B.; Jones Junior, J.; da Silva, F.M. The Povarov reaction as a versatile strategy for the preparation 1,2,3,4-tetrahydroquinoline derivatives: An overview. Curr. Org. Synth. 2016, 13, 157–175. [Google Scholar] [CrossRef]
- Marques Rezende, T.R.; Santana Varejão, J.O.; Lacerda de Almeida Sousa, A.L.; Bonilla Castañeda, S.M.; Fernandes, S.A. Tetrahydroquinolines by the multicomponent Povarov reaction in water: Calix[n]arene-catalysed cascade process and mechanistic insights. Org. Biomol. Chem. 2019, 17, 2913–2922. [Google Scholar] [CrossRef]
- Sanz-Vidal, Á.; Miró, J.; Sánchez-Roselló, M.; Del Pozo, C.; Fustero, S. Gold-Catalyzed Povarov-Type Reaction of Fluorinated Imino Esters and Furans. J. Org. Chem. 2016, 81, 6515–6524. [Google Scholar] [CrossRef]
- Mahi, M.A.; Mekelleche, S.M.; Benchouk, W.; Aurell, M.J.; Domingo, L.R. Theoretical study of the regio- and stereoselectivity of the intramolecular Povarov reactions yielding 5H-chromeno[2,3-c] acridine derivatives. RSC Adv. 2016, 6, 15759–15769. [Google Scholar] [CrossRef]
- Peñaranda Gómez, A.; Rodríguez Bejarano, O.; Kouznetsov, V.V.; Ochoa-Puentes, C. One-Pot Diastereoselective Synthesis of Tetrahydroquinolines from Star Anise Oil in a Choline Chloride/Zinc Chloride Eutectic Mixture. ACS Sustain. Chem. Eng. 2019, 7, 18630–18639. [Google Scholar] [CrossRef]
- Taylor, J.E.; Bull, S.D. N-Acylation reactions of amines. In Comprehensive Organic Synthesis II; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 427–478. [Google Scholar]
- Li, J.S.; Da, Y.D.; Chen, G.Q.; Yang, Q.; Li, Z.W.; Yang, F.; Huang, P.M. Solvent-, and Catalyst–free Acylation of Anilines with Meldrum’s Acids: A Neat Access to Anilides. ChemistrySelect 2017, 2, 1770–1773. [Google Scholar] [CrossRef]
- Chapman, R.S.; Tibbetts, J.D.; Bull, S.D. 1,1-Diacyloxy-1-phenylmethanes as versatile N-acylating agents for amines. Tetrahedron 2018, 74, 5330–5339. [Google Scholar] [CrossRef]
- Rodríguez Enciso, D.A.; Puerto Galvis, C.E.; Kouznetsov, V.V. 2-(4-Chlorophenyl)-4-(3,4-dimethoxy-phenyl)-6-methoxy-3-methylquinoline. Molbank 2022, 2022, M1383. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla-Castañeda, S.M.; Villamizar-Mogotocoro, A.F.; Kouznetsov, V.V. [4-(3,4-Dimethoxyphenyl)-3,6-dimethyl-2-phenyl-3,4-dihydroquinolin-1(2H)-yl)](furan-2-yl)methanone. Molbank 2022, 2022, M1413. https://doi.org/10.3390/M1413
Bonilla-Castañeda SM, Villamizar-Mogotocoro AF, Kouznetsov VV. [4-(3,4-Dimethoxyphenyl)-3,6-dimethyl-2-phenyl-3,4-dihydroquinolin-1(2H)-yl)](furan-2-yl)methanone. Molbank. 2022; 2022(3):M1413. https://doi.org/10.3390/M1413
Chicago/Turabian StyleBonilla-Castañeda, Sandra M., Andrés F. Villamizar-Mogotocoro, and Vladimir V. Kouznetsov. 2022. "[4-(3,4-Dimethoxyphenyl)-3,6-dimethyl-2-phenyl-3,4-dihydroquinolin-1(2H)-yl)](furan-2-yl)methanone" Molbank 2022, no. 3: M1413. https://doi.org/10.3390/M1413
APA StyleBonilla-Castañeda, S. M., Villamizar-Mogotocoro, A. F., & Kouznetsov, V. V. (2022). [4-(3,4-Dimethoxyphenyl)-3,6-dimethyl-2-phenyl-3,4-dihydroquinolin-1(2H)-yl)](furan-2-yl)methanone. Molbank, 2022(3), M1413. https://doi.org/10.3390/M1413