3,6-Dihydro-5H-pyrazolo [4′,3′:5,6]pyrano[3,4-b]indol-5-one
Abstract
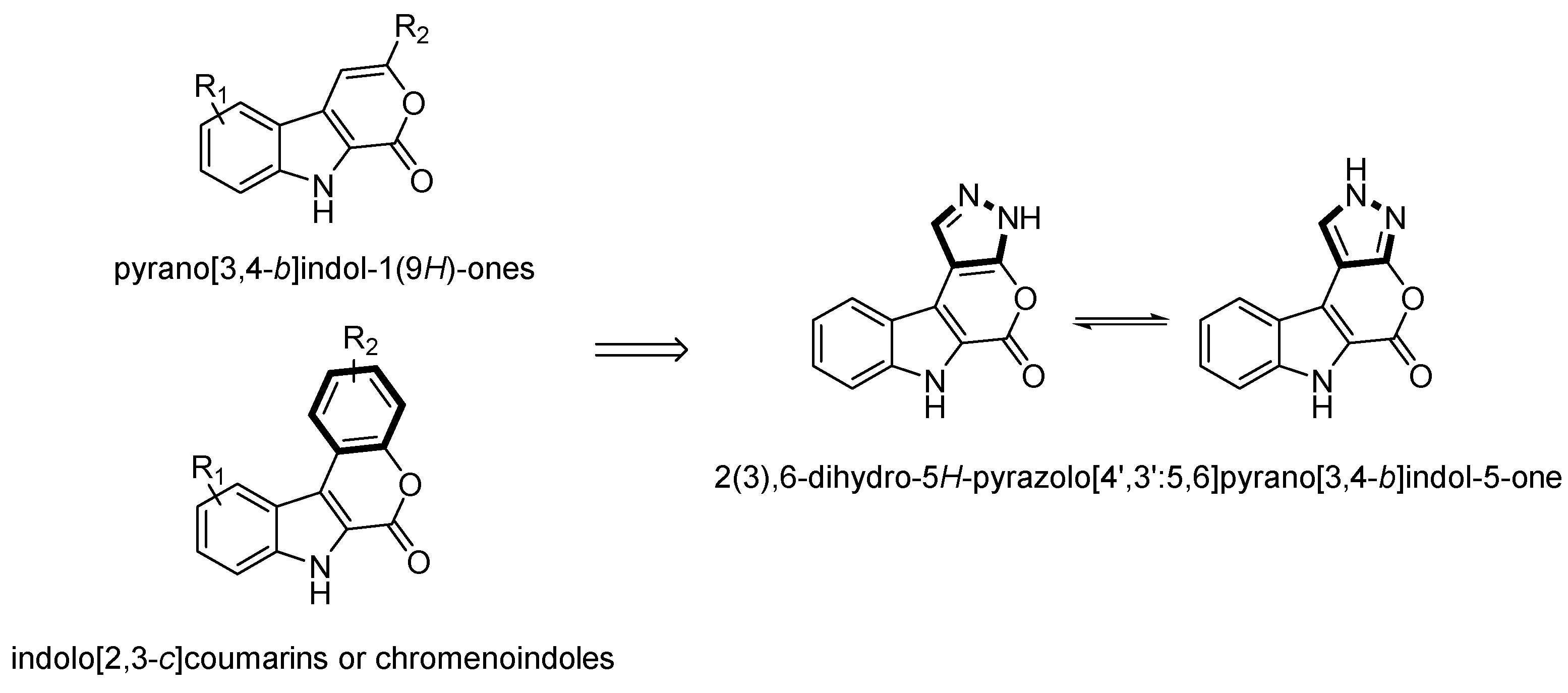
:1. Introduction
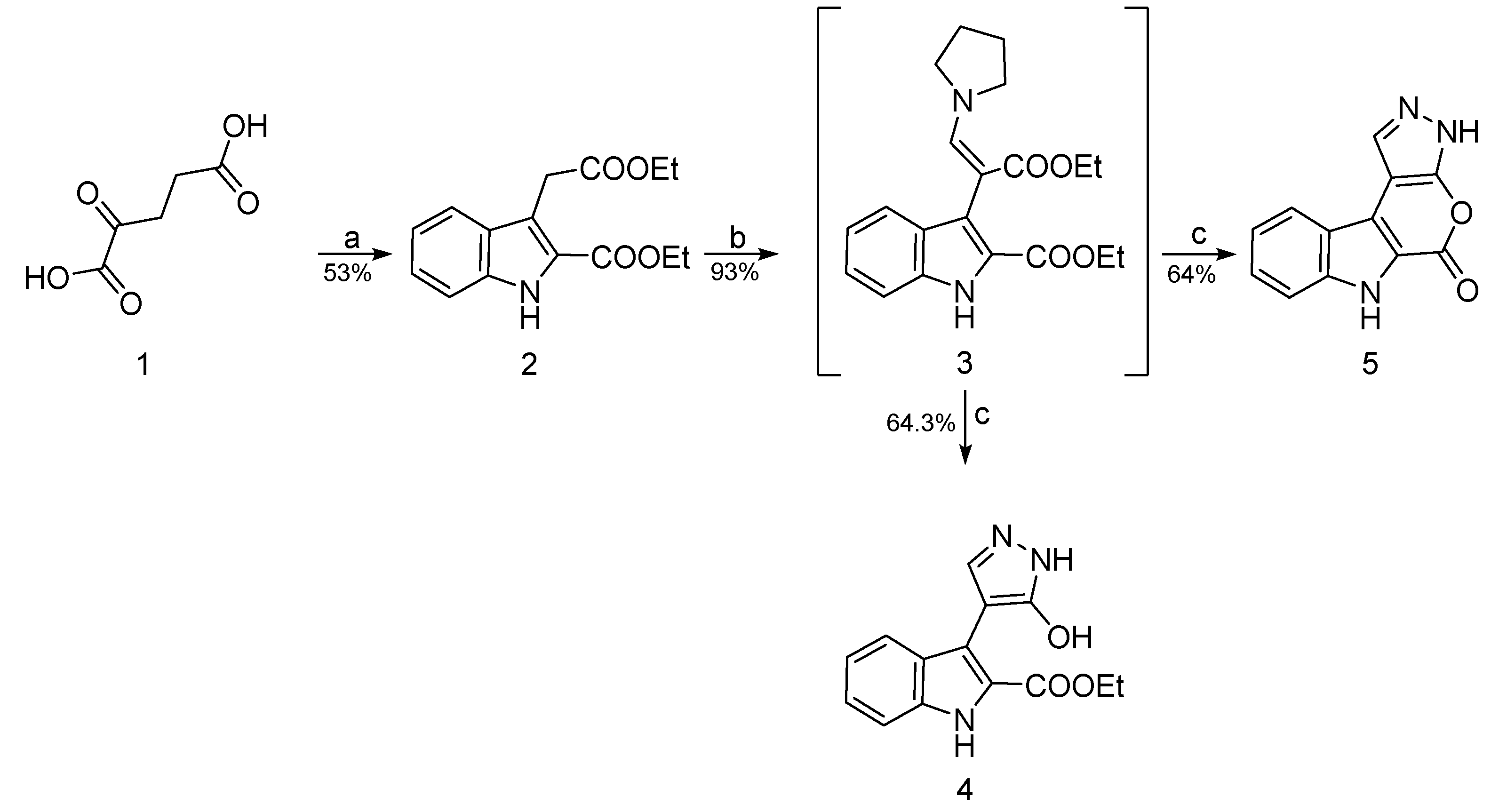
2. Results and Discussion
3. Materials and Methods
3.1. General
3.1.1. Ethyl 3-(2-ethoxy-2-oxoethyl)-1H-indole-2-carboxylate (2)
3.1.2. Ethyl 3-(3-ethoxy-3-oxo-1-(pyrrolidin-1-yl)prop-1-en-2-yl)-1H-indole-2-carboxylate (3)
3.1.3. Ethyl 3-(3-hydroxy-1H-pyrazol-4-yl)-1H-indole-2-carboxylate (4)
3.1.4. 3,6-Dihydro-5H-pyrazolo [4′,3′:5,6]pyrano [3,4-b]indol-5-one (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Praveen, C.; Ayyanar, A.; Perumal, P.T. Gold(III) chloride catalyzed regioselective synthesis of pyrano[3,4-b]indol-1(9H)-ones and evaluation of anticancer potential towards human cervix adenocarcinoma. Bioorg. Med. Chem. Lett. 2011, 21, 4170–4173. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikrishna, D.; Godugu, C.; Dubey, P.K. A Review on Pharmacological Properties of Coumarins. Mini Rev. Med. Chem. 2018, 18, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman. Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, C.-X.; Chen, W.-W.; Xu, B.; Xu, M.-H. Synthesis of indolo[2,3-c]coumarins and indolo[2,3-c]quinolinones via microwave-assisted base-free intramolecular cross dehydrogenative coupling. Tetrahedron 2019, 75, 1605–1611. [Google Scholar] [CrossRef]
- Andersen, R.J.; Faulkner, D.J.; He, C.-H.; Van Duyne, G.D.; Clardy, J. Metabolites of the marine prosobranch mollusk Lamellaria sp. J. Am. Chem. Soc. 1985, 107, 5492–5495. [Google Scholar] [CrossRef]
- Pia, D.; Albericio, F.; Alvare, M. Recent Advances in Lamellarin Alkaloids: Isolation, Synthesis and Activity. Anti Cancer Agents Med. Chem. 2008, 8, 746–760. [Google Scholar]
- Kang, H.; Fenical, W. Ningalins A−D: Novel Aromatic Alkaloids from a Western Australian Ascidian of the Genus Didemnum. J. Org. Chem. 1997, 62, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.M.; Dappen, J.M.S.; Cheng, B.K.; Cordi, A.A.; Biesterfeldt, J.P.; Hood, W.F.; Monahan, J.B. Novel Indole-2-carboxylates as Ligands for the Strychnine-Insensitive N-Methyl-D-aspartate-Linked Glycine Receptor. J. Med. Chem. 1991, 34, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry, 5th ed.; Blackwell Publishing Ltd.: Oxford, UK, 2009; pp. 402–405. [Google Scholar]
- Elguero, H.; Katritzky, A.R.; Denisco, O.V. Prototropic Tautomerism of Heterocycles: Heteroaromatic Tautomerism-General Overview and Methodology. Adv. Heterocycl. Chem. 2000, 76, 1–84. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalampaliki, A.D.; Kanellopoulou, S.; Kostakis, I.K. 3,6-Dihydro-5H-pyrazolo [4′,3′:5,6]pyrano[3,4-b]indol-5-one. Molbank 2022, 2022, M1412. https://doi.org/10.3390/M1412
Kalampaliki AD, Kanellopoulou S, Kostakis IK. 3,6-Dihydro-5H-pyrazolo [4′,3′:5,6]pyrano[3,4-b]indol-5-one. Molbank. 2022; 2022(3):M1412. https://doi.org/10.3390/M1412
Chicago/Turabian StyleKalampaliki, Amalia D., Sofia Kanellopoulou, and Ioannis K. Kostakis. 2022. "3,6-Dihydro-5H-pyrazolo [4′,3′:5,6]pyrano[3,4-b]indol-5-one" Molbank 2022, no. 3: M1412. https://doi.org/10.3390/M1412
APA StyleKalampaliki, A. D., Kanellopoulou, S., & Kostakis, I. K. (2022). 3,6-Dihydro-5H-pyrazolo [4′,3′:5,6]pyrano[3,4-b]indol-5-one. Molbank, 2022(3), M1412. https://doi.org/10.3390/M1412






