Abstract
Zwitterionic polymers emerge as very useful materials for applications in antifouling surfaces. The properties of these polymers can be tuned by variations of the chemical structure of the corresponding monomer. In this study, two zwitterionic ammonium sulfonate monomers, bearing hydroxyl function and styrenic polymerizable groups, were prepared in two steps. The two monomers were obtained using 4-vinylbenzyl chloride as the key precursor. The zwitterionic monomers were characterized by 1H NMR, 13C NMR, IR spectroscopy, and high-resolution mass spectrometry (HRMS). These two monomers enable the preparation of novel zwitterionic polymers with enhanced hydrophilicity, due to the presence of a hydroxyl group.
1. Introduction
Zwitterionic polymers (or polyzwitterions) exhibit a structure with an equimolar number of anionic and cationic groups on their repeat units. These polymers are mainly found in two forms, with the charges located either on the pendent side chains of the monomer unit or along the polymer backbone (Figure 1) [1,2]. The main application of zwitterionic polymers is the preparation of nonfouling materials and surfaces, due to the repelling of bacterial adhesion through polymer hydration [3,4,5].
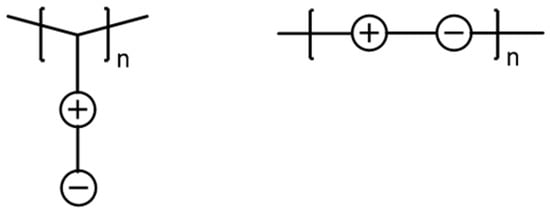
Figure 1.
Examples of possible distributions of ionic groups within zwitterionic polymers (schematic representation).
The chemical diversity of zwitterionic polymers comes exclusively from the ionic groups in the monomer. The main cations are obtained by the alkylation of a nitrogen or phosphorus atom to generate quaternary ammonium, pyridinium, imidazolium, or phosphonium groups. The anions used are carboxylate, sulfonate, or phosphate groups. To prepare antifouling surfaces, methacrylate and acrylamide are the most common polymerizable groups used for zwitterionic monomers [2,3]. However, some studies reported the use of a styrenic polymerizable group [6,7]. Very recently, T. Emrick et al., described the preparation of new zwitterionic monomers using 4-vinylbenzyl sultone as a precursor to incorporating ammonium sulfonate units [6]. Indeed, numerous studies reported in the literature have shown that excellent antibacterial and anti-fouling properties were achieved by combination of ammonium cationic parts with sulfonate anionic moiety [8,9].
In this context, we describe the synthesis of two styrenic zwitterionic monomers bearing imidazolium or ammonium cation linked to a sulfonate anion. In these monomers, a hydroxyl function could intensify the hydrophilic character of the corresponding polymers and enhance their anti-fouling properties [10].
2. Results and Discussion
Recently, our team has developed new efficient approaches to functionalized ionic monomers for their use in epoxy resins [11,12]. Here, we describe a simple two-step sequence from imidazole or dimethylamine and sodium-3-chloro-2-hydroxypropane-1-sulfonate for the preparation of zwitterionic monomers bearing a styrenic moiety (Scheme 1). In this strategy, we used 4-vinylbenzylchloride as an inexpensive and, particularly reactive, starting material. Firstly, we added a stoechiometic amount of imidazole in the presence of powdered sodium hydroxide (1 eq) in THF. After 48 h at room temperature, the crude mixture was poured in water, and 4-vinylbenzylimidazole 1 was extracted by dichloromethane to provide an excellent yield of 96%. NMR analysis confirmed the alkylated imidazole derivative 1, with no purification required at this step. The quaternarization of the imidazole 1 was performed by reaction with sodium 3-chloro-2-hydroxypropane-1-sulfonate hemihydrate in a mixture of water/methanol (1/1). To optimize this second alkylation, we dissolved the sulfonate in water at room temperature. This aqueous solution was added to imidazole 1 in methanol, and the resulting mixture was heated at 90 °C for 72 h. With one-and-one-half eq of imidazole 1, a poor conversion of 60% was obtained after 72 h. After the reaction, we tested the purification of the crude mixture using Amberlite MB-6113 H+/OH− mixed resin. To this end, the crude monomer was precipitated in ethyl acetate and dissolved in water. Then, the mixed-bed ion-exchange resin was added, and the suspension was stirred at room temperature until the disappearance of sodium 3-chloro-2-hydroxypropane-1-sulfonate (followed by 1H NMR spectroscopy). The resin was filtered. Then, water was removed under reduced pressure; finally, the residue was dried in vacuo. This protocol afforded a purified product, but a significant loss of mass was observed.
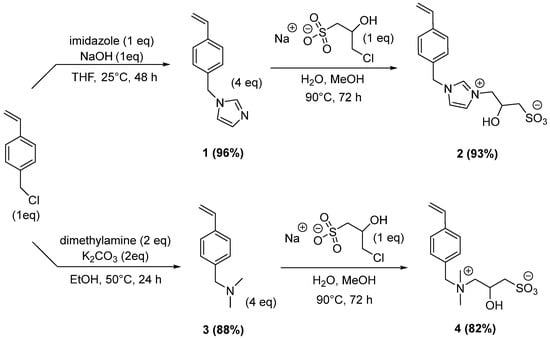
Scheme 1.
Synthesis of zwitterionic salts 2 and 4 from 4-vinylbenzylchloride.
In a second study, we used an excess of imidazole 1 to completely consume the 3-chloro-2-hydroxypropane-1-sulfonate and facilitate the purification of the final zwitterion. With four equivalents (eq) of imidazole 1, the conversion reached 95% after 72 h at room temperature. The addition of a large excess of imidazole 1 (6 eq) had no influence on the kinetics of this reaction. In both cases, the precipitation of the zwitterionic compound in ethyl acetate led to the pure product in 93% yield. Thermal behavior and stability of this monomer were investigated by TGA. Until 300 °C, the weight loss was negligible. A weight loss equal to 5% was obtained at 357 °C. Using the first derivative of the TGA as a function of temperature, a maximum rate of decomposition was obtained at 385 and 482 °C.
To complete the previous sequence, we used dimethylamine to provide a new cationic moiety 4 (Scheme 1). This nucleophile is reacted with 4-vinylbenzylchloride to generate tertiary amine 3 in 88% yield. In this case, the amine 3 was purified by column chromatography on silica gel with CH2Cl2/MeOH (90:10) as the eluent. Finally, the zwitterionic compound was synthesized by quaternarization with sodium-3-chloro-2-hydroxypropane-1-sulfonate hemihydrate under similar conditions, as previously described in the case of imidazole 1. The ammonium was isolated by precipitation in acetonitrile and filtration to provide the zwitterionic salt 4 in 82% yield.
Various tests of solubility have been carried out with zwitterionic salts 2 and 4. We used a large number of usual organic solvents, such as CH2Cl2, MeCN, MeOH, and DMF, which do not solubilize the two compounds. In DMSO and water, a poor solubility was observed in both cases. To improve the hydrosolubility of these salts, we added NaCl (four eq for 2 and two eq for 4) to obtain a homogeneous mixture.
3. Materials and Methods
3.1. Materials
All reagents were obtained from commercial suppliers and used without further purification: imidazole (TCI), dimethylamine (40 wt% in H2O, Sigma Aldrich, Saint Quentin Fallavier, France), 4-vinylbenzylchloride (90%, contains 0.1% total stabilizer, Sigma Aldrich), and sodium 3-chloro-2-hydroxypropane-1-sulfonate hemihydrate (Alfa Aesar, Kandel, Germany). 1H NMR and 13C NMR were recorded using 500 Mhz apparatus in an appropriate deuterated solvent (CDCl3, D2O, and DMSO-d6 from Eurisotop, St. Aubin, France). All chemical shifts δ are reported in parts in ppm (part per million), relative to internal tetramethylsilane; coupling constants (J) are indicated in Hertz (Hz). Abbreviations for signal coupling are as follows: s = singlet; d = doublet; dd = doublet of doublets; t = triplet; q = quartet; m = multiplet.
Attenuated total reflection infrared (ATR IR) spectra were recorded using a Perkin Elmer 16 PC FTIR ATR spectrometer.
High-resolution mass spectra were recorded on Acquity UPLC H-Class Xevo G2-XS QTof (WATERS) by electrospray ionization (ESI).
Thermogravimetric analyses (TGA) were conducted on Perkin Elmer TGA Pyris 1, with a heating rate of 20 °C/min under a nitrogen atmosphere.
3.2. Synthesis of Imidazole 1
Imidazole (3.6 g, 50 mmol, 1.0 eq) was dissolved in 50 mL THF and added dropwise to a suspension of NaOH (3.16 g, 50 mmol, 1.0 eq) in THF. The resulting mixture was refluxed for 2 h. After cooling, 4-vinylbenzylchloride (8.03 g, 50 mmol, 1 eq) was slowly added, and the reaction mixture was stirred at room temperature for 48 h. The resulting mixture was dissolved in water, and the product was extracted with CH2Cl2 (3 × 50 mL). The combined organic layers were dried on MgSO4 and filtered and concentrated under reduced pressure to provide the 1-(4-vinylbenzyl)imidazole (8.90 g, 96%), which was pure enough to undergo the next step. 1H and 13C NMR spectra were in agreement with the literature data [13].
3.3. Synthesis of Zwitterion 2
Sodium-3-chloro-2-hydroxypropane-1-sulfonate hemihydrate (1.4 g, 6.7 mmol, and 1.0 eq) was dissolved in H2O (20 mL) and added to 1-(4-vinylbenzyl)imidazole (5 g, 27 mmol, and 4.0 eq) in MeOH (24 mL). The reaction mixture was stirred at 90 °C for 72 h. The mixture was concentrated under reduced pressure. The product was precipitated in ethyl acetate, filtered, and washed with ethyl acetate. The zwitterion was obtained as a white powder (2.02 g, 93%).
1H NMR (500 MHz, D2O) δ 3.01 (d, J = 6.1 Hz, 2H), 4.18 (dd, J = 14.2 and 7.9 Hz, 1H), 4.28–4.31 (m, 1H), 4.39 (dd, J = 14.2 and 2.8 Hz, 1H), 5.24 (d, J = 11.1 Hz, 1H), 5.28 (s, 2H), 5.77 (d, J = 17.7 Hz, 1H), 6.69 (dd, J = 17.7 and 11.1 Hz, 1H), 7.27 (d, J = 8.2 Hz, 2H), 7.39 (m, 1H), 7.43–7.45 (m, 3H), and 8.87 (s, 1H) (Supplementary Figure S1).
13C NMR (125 MHz, D2O) δ 138.5, 136.0, 133.0, 129.0, 127.0, 123.5, 122.4, 115.5, 66.0, 54.01, 53.8, and 52.7 (Supplementary Figure S2).
IR (neat) cm−1 3671, 3342, 3086, 2980, 1552, 1410, 1191, 1155, and 1039 (Supplementary Figure S5).
HRMS m/z (ESI): calcd, for C15H17N2O4S [M − H]−: 321.0909, found: 321.0905 (Supplementary Figure S7).
TGA: T5% = 357 °C, T20% = 407 °C, and T50% = 611 °C.
3.4. Synthesis of Amine 3
The 4-vinylbenzylchloride (10 g, 65 mmol, and 1.0 eq) was added to a dimethylamine aqueous solution (5.9 g, 131 mmol, and 2.0 eq) and K2CO3 (18.11 g, 131 mmol, and 2.0 eq) in ethanol (60 mL). The mixture was degassed with argon and then heated to 50 °C. After 24 h, the solvent was removed under vacuum to obtain the crude product. The amine was purified by silica gel column chromatography, with CH2Cl2 /MeOH (90:10) as the eluent, to obtain a colorless liquid (9.3 g, 88%). 1H and 13C NMR spectra are in agreement with the literature data [14].
3.5. Synthesis of Zwitterion 4
Sodium-3-chloro-2-hydroxypropane-1-sulfonate hemihydrate (1.6 g, 7.8 mmol, and 1.0 eq) was dissolved in H2O (20 mL) and added to N-(4-vinylbenzyl)dimethylamine (5 g, 31 mmol, and 4.0 eq) in MeOH (20 mL). The reaction mixture was stirred at 90 °C for 72 h. The mixture was concentrated under reduced pressure. The product was precipitated in acetonitrile, filtered, and washed with acetonitrile to provide zwitterionic salt 4 (1.9 g, 82%) as a white powder.
m.p. = 211 °C.
1H NMR (500 MHz, D2O) δ 3.01–3.06 (m, 8H), 3.41 (dd, J = 14.0 and 9.7 Hz, 1H), 3.56 (dd, J = 14.0 and 1.3 Hz, 1H), 4.47 (A(AB), JAB = 13.0 Hz, 1H), 4.51 (B(AB) JAB = 13.0 Hz, 1H), 4.64–4.67 (m, 1H), 5.32 (d, J = 11.0 Hz, 1H), 5.84 (d, J = 17.7 Hz, 1H), 6.73 (dd, J = 17.7 and 11.0 Hz, 1H), 7.26 (d, J = 8.2 Hz, 2H), and 7.51 (d, J = 8.2 Hz, 2H) (Supplementary Figure S3).
13C NMR (125 MHz, D2O) δ 139.8, 135.8, 133.5, 126.7, 126.3, 116.4, 69.6, 67.5, 62.7, 55.3, 50.9, and 50.2 (Supplementary Figure S4).
IR (neat) cm−1. 3296, 3027, 2978, 1630, 1480, 1411, 1194, and 1165 (Supplementary Figure S6).
HRMS m/z (ESI): calcd. for C14H20NO4S [M − H]−: 298.1113, found: 298.1109.
4. Conclusions
We have synthesized two styrenic monomers bearing a zwitterionic group (imidazolium or ammonium, corresponding to the cationic moiety and sulphonate for the anionic moiety), in association with a hydroxyl function. From 4-vinylbenzylchloride, as starting material, salts 2 and 4 were obtained in high overall yield of 89.5% and 72.5%, respectively. These monomers can be polymerized by conventional or reversible-deactivation radical polymerizations to produce hydrophilic and/or antifouling materials. They could be used for surface modification in medical equipment (stents, catheter, and dental implantology) and micelles preparation for drug delivery systems.
Supplementary Materials
Figure S1: 1H NMR spectrum of monomer 2 recorded in D2O, Figure S2: 13C NMR spectrum of monomer 2 recorded in D2O, Figure S3: 1H NMR spectrum of monomer 4 recorded in D2O, Figure S4: 13C NMR spectrum of monomer 4 recorded in D2O, Figure S5: FTIR spectrum of monomer 2, Figure S6: FTIR spectrum of monomer 4, Figure S7: Thermogravimetric analysis (TGA) and derivative thermogravimetric curves of monomer 2.
Author Contributions
Conceptualization, methodology, and validation, B.L. and J.B.; formal analysis and investigation, J.B.-H.-S.; writing—original draft preparation, B.L. and J.B.; writing—review and editing, project administration, funding acquisition and supervision, B.L., J.B., J.R. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Ministère de l’Enseignement Supérieur et de la Recherche Scientifique Tunisien–Université de Carthage” (fellowships to J.B.-H.-S.), Ministry of Higher Education and Research in France, “Region Basse Normandie”, LABEX SynOrg (ANR11-LABX-0029), and European FEDER.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Romuald Herbinet for the TGA analyses.
Conflicts of Interest
The authors report no declarations of interest.
References
- Laschewsky, A. Structures and Synthesis of Zwitterionic Polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002, 102, 4177–4189. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Jiang, S. Integrated Antimicrobial and Nonfouling Zwitterionic Polymer. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Jiang, S. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z. Surface Hydration and Antifouling Activity of Zwitterionic Polymers. Langmuir 2022, 38, 4483–4489. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.U.; Seong, H.G.; Margossian, K.O.; Bishop, L.; Russell, T.P.; Muthkumar, M.; Emrick, T. Zwitterionic Ammonium Sulfonate Polymers: Synthesis and Properties in Fluids. Macromol. Rapid Commun. 2022, 43, 2100678. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, H.; Yamada, S.; Endo, T. Preparation of zwitterionic polymer based on L-cysteine for recovery application of precious metals. RSC Adv. 2016, 6, 108689–108696. [Google Scholar] [CrossRef]
- Zhao, W.; Ye, Q.; Hu, H.; Wang, X.; Zhou, F. Grafting zwitterionic polymer brushes via electrochemical surface-initiated atomic-transfer radical polymerization for anti-fouling applications. J. Mater. Chem. B 2014, 2, 5352–5357. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; He, B.; Zhang, Y.; Liu, S.; Zhou, F. Grafting Robust Thick Zwitterionic Polymer Brushes via Subsurface-Initiated Ring-Opening Metathesis Polymerization for Antimicrobial and Anti-Biofouling. ACS Appl. Mater. Interfaces 2019, 11, 39171–39178. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Zhang, M.; Zhou, N.; Wu, F.; Sun, B.; Shen, J. Synthesis and characterization of a novel antibacterial material containing poly(sulfobetaine) using reverse atom transfer radical polymerization. RSC Adv. 2018, 8, 33000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livi, S.; Chardin, C.; Lins, L.C.; Halawani, N.; Pruvost, S.; Duchet-Rumeau, J.; Gerard, J.F.; Baudoux, J. From Ionic Liquid Epoxy Monomer to Tunable Epoxy—Amine Network: Reaction Mechanism and Final Properties. ACS Sustain. Chem. Eng. 2019, 7, 3602–3613. [Google Scholar] [CrossRef]
- Kui, T.; Chardin, C.; Rouden, J.; Livi, S.; Baudoux, J. Sulfonates as Versatile Structural Counterions of Epoxidized Salts. ChemSusChem 2022, e202200198. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sun, Q.; Fu, Y.; Song, L.; Liang, J.; Xu, X.; Wang, H.; Li, J.; Tu, S.; Lu, X.; et al. Sponge-like quaternary ammonium-based poly(ionic liquid)s: Toward high CO2 capture and efficient cycloaddition at mild conditions. J. Mater. Chem. A 2017, 5, 25594–25600. [Google Scholar] [CrossRef]
- Dan, M.; Su, Y.; Xiao, X.; Li, S.; Zhang, W. A New Family of Thermo-Responsive Polymers Based on Poly[N-(4- vinylbenzyl)-N,N-dialkylamine]. Macromolecules 2013, 46, 3137–3146. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).