Abstract
We elaborated a convenient one-step approach for the synthesis of previously unknown 2-(5-acetyl-7-methoxy-2-(4-methoxyphenyl)benzofuran-3-yl)acetic acid. The suggested protocol includes the multicomponent reaction of acetovanillone, 4-methoxyphenylglyoxal and Meldrum’s acid. We have demonstrated that the considered reaction is a one-pot telescoped process including the preliminary condensation of the components in MeCN followed by acid-catalyzed cyclization. The structure of the synthesized product was confirmed by 1H, 13C-NMR spectroscopy and high-resolution mass-spectrometry.
1. Introduction
Acetovanillone (Apocynin) is a natural organic compound structurally related to vanillin found in plant sources [1,2]. Acetovanillone has a wide spectrum of biological activity. For example, apocynin can influence the immune system through the inhibition of NADPH oxidase [3,4,5,6,7,8,9,10,11]. Besides that, acetovanillone is used as an anti-arthritic [12,13] and anti-asthmatic agent [14]. Additionally, apocynin can be employed for the treatment of bowel diseases [15] and atherosclerosis [16]. Finally, it was shown that this compound displays significant activity against amyotrophic lateral sclerosis [17]. In this regard, the preparation of synthetic derivatives of acetovanillone, which may also have various biological activities, is of considerable interest.
Previously, we proposed a general method for the preparation of condensed furylacetic acids based on a multicomponent reaction of hydroxyl derivatives with arylglyoxals and Meldrum’s acid [18,19,20,21,22,23,24]. It should be noted that the considered method allows one to synthesize the wide range of furylacetic acids from the diverse phenols. We assumed that the presented approach could be applied to obtain the condensed acetovanillone derivatives.
2. Results and Discussion
In the present paper, we describe a multicomponent reaction of acetovanillone 1, 4-methoxyphenylglyoxal 2 and Meldrum’s acid 3 leading to 2-(5-acetyl-7-methoxy-2-(4-methoxyphenyl)benzofuran-3-yl)acetic acid 4 unknown in the literature (Scheme 1). It was shown that the studied reaction was a one-pot telescoped two stage process. The first step involves the condensation of the starting components in acetonitrile in the presence of triethylamine. Note that the initial stage of the process can be carried out under mild conditions at room temperature due to the high solubility of acetovanillone 1. In this case, the reaction proceeds rather slowly, and a complete conversion of apocynin 1 was observed in 48 h. At the same time, our attempts to accelerate the process by increasing the temperature of the first stage led to a significant decrease in the yield of the target product 4. It should be mentioned that the final step of the reaction involves intramolecular cyclization in a mixture of hydrochloric and acetic acids. As was shown previously, these conditions are optimal for the last stage of the process [18,19,20,21,22,23,24]. Thus, the presented approach allows one to obtain the target product 4 in a 62% yield.
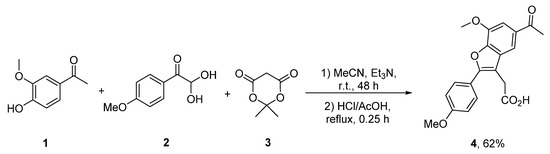
Scheme 1.
Synthesis of 2-(5-acetyl-7-methoxy-2-(4-methoxyphenyl)benzofuran-3-yl)acetic acid 4.
The assumed pathway of the considered reaction is shown in Scheme 2. At first, the condensation of 4-methoxyphenylglyoxal 2 with Meldrum’s acid 3 results in unstable Michael acceptor A. Then, the interaction of acetovanillone anion B with aroylmethylene derivative A leads to adduct D. At the next step, intermediate D cyclizes into γ-ketoacid F with the elimination of acetone and CO2 molecules. Finally, the target furylacetic acid 4 is formed as a result of an acid-catalyzed intramolecular cyclization of compound F.
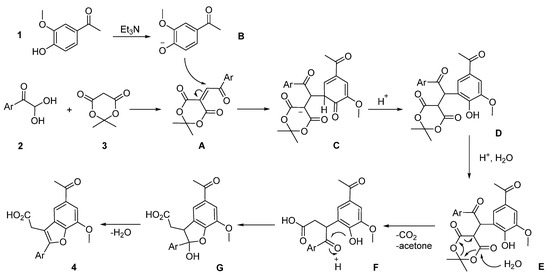
Scheme 2.
The assumed reaction pathway for the formation of compound 4.
The synthesized 2-(5-acetyl-7-methoxy-2-(4-methoxyphenyl)benzofuran-3-yl)acetic acid 4 is the solid crystalline compound, whose structure was confirmed by 1H, 13C NMR spectroscopy and high-resolution mass-spectrometry. 1H NMR spectra of the product 4 contain characteristic signals of the protons of the carboxymethylene fragment in the region δ 3.89 ppm and 12.57 ppm. The remaining signals are also in good agreement with the presented structure.
In summary, we suggested an efficient method for the modification of naturally occurring acetovanillone. The considered approach allows one to synthesize the previously unknown 2-(5-acetyl-7-methoxy-2-(4-methoxyphenyl)benzofuran-3-yl)acetic acid. The studied reaction is based on the multicomponent condensation of acetovanillone, 4-methoxyphenylglyoxal and Meldrum’s acid. The advantages of this protocol are the application of readily available starting compounds, an atom economy and an easy work-up procedure, which can avoid chromatographic purification. The structure of the synthesized product was confirmed by 1H, 13C-NMR spectroscopy and high-resolution mass-spectrometry.
3. Materials and Methods
All starting chemicals and solvents were commercially available and were used as received. NMR spectra were recorded with Bruker DRX 300 (300 MHz) spectrometers (Billerica, MA, USA) in DMSO-d6. Chemical shifts (ppm) were given relative to solvent signals (DMSO-d6: 2.50 ppm (1H NMR) and 39.52 ppm (13C NMR)). High-resolution mass spectra (HRMS) were obtained on a Bruker micrOTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (ESI). The melting points were determined on a Kofler hot stage (Dresden, Germany). IR spectra were recorded on a Bruker ALPHA (Santa Barbara, CA, USA) spectrophotometer in a KBr pellet.
Experimental Procedure for the Synthesis of 2-(5-Acetyl-7-methoxy-2-(4-methoxyphenyl)benzofuran-3-yl)acetic Acid 4
A mixture of acetovanillone 1 (3 mmol, 0.5 g), 4-methoxyphenylglyoxal hydrate 2 (5 mmol, 0.91 g), Meldrum’s acid 3 (6 mmol, 0.86 g), and Et3N (7 mmol, 1 mL) in 6 mL of MeCN was kept for 48 h at room temperature. Then, 2 mL AcOH was added, and the reaction mixture was evaporated in vacuo. 3 mL of conc. HCl and 5 mL of AcOH were added to the residue, and the solution was refluxed for 15 min. The resulting mixture was stirred for 2 h at room temperature and left overnight. The formed precipitate was collected by filtration and washed with 50% aqueous AcOH (3 × 7 mL). To remove traces of HCl and AcOH, the precipitate was kept for 24 h in water (50 mL) at room temperature, collected by filtration, and washed with water (3 × 10 mL). Beige powder; yield 62% (0.66 g, 1.9 mmol); mp 193–195 °C. 1H NMR (300 MHz, DMSO-d6) δ 12.57 (br.s, 1H), 7.96 (s, 1H), 7.72 (d, J = 8.8 Hz, 2H), 7.45 (s, 1H), 7.12 (d, J = 8.7 Hz, 2H), 4.02 (s, 3H), 3.89 (s, 2H), 3.83 (s, 3H), 2.64 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 197.25, 171.84, 159.93, 153.25, 144.77, 144.59, 133.36, 131.57, 128.26, 121.85, 114.59, 114.18, 109.34, 106.01, 55.99, 55.33, 29.94, 26.79. IR spectrum (KBr), ν, cm−1: 3418, 3089, 3056, 3000, 2980, 2945, 2841, 2706, 2596, 2361, 2341, 2042, 1852, 1720, 1646, 1615, 1589, 1512, 1481, 1466, 1422, 1391, 1321, 1302, 1260, 1218, 1178, 1088, 1053, 1026. HRMS (ESI-TOF) m/z: [M + H]+ Calcld for C20H18O6 355.1176; Found 355.1173.
Supplementary Materials
The following are available online: copies of 1H, 13C-NMR, mass and IR spectra for compound 4. Figure S1: 1H NMR spectrum (300 MHz) of compound 4 in DMSO-d6; Figure S2: 13C {1H} NMR spectrum (75 MHz) of compound 4 in DMSO-d6; Figure S3: HRMS for compound 4; Figure S4: IR spectrum for compound 4.
Author Contributions
A.N.K.—conceptualization, synthesis, spectroscopic analysis and writing the manuscript. B.V.L.—conceptualization, synthesis, spectroscopic analysis and writing the manuscript. V.G.M.—conceptualization, synthesis, spectroscopic analysis and writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for the compounds presented in this study are available in the Supplementary Materials of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Debnath, P.; Rathore, S.; Walia, S.; Kumar, M.; Devi, R.; Kumar, R. Picrorhiza Kurroa: A Promising Traditional Therapeutic Herb from Higher Altitude of Western Himalayas. J. Herb. Med. 2020, 23, 100358. [Google Scholar] [CrossRef]
- Kumar, S.; Patial, V.; Soni, S.; Sharma, S.; Pratap, K.; Kumar, D.; Padwad, Y. Picrorhiza Kurroa Enhances β-Cell Mass Proliferation and Insulin Secretion in Streptozotocin Evoked β-Cell Damage in Rats. Front. Pharmacol. 2017, 8, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Zang, D.-W.; Shan, W.; Guo, A.-C.; Wu, J.-P.; Wang, Y.-J.; Wang, Q. Synthesis and Evaluations of Novel Apocynin Derivatives as Anti-Glioma Agents. Front. Pharmacol. 2019, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Petrônio, M.; Zeraik, M.; Fonseca, L.; Ximenes, V. Apocynin: Chemical and Biophysical Properties of a NADPH Oxidase Inhibitor. Molecules 2013, 18, 2821–2839. [Google Scholar] [CrossRef] [Green Version]
- Stefanska, J.; Pawliczak, R. Apocynin: Molecular Aptitudes. Mediat. Inflamm. 2008, 2008, 106507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, A.L.; Hawkes, J.; Wright, H.L.; Moots, R.J.; Edwards, S.W. APPA (Apocynin and Paeonol) Modulates Pathological Aspects of Human Neutrophil Function, without Supressing Antimicrobial Ability, and Inhibits TNFα Expression and Signalling. Inflammopharmacology 2020, 28, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Montes-Rivera, J.O.; Tamay-Cach, F.; Quintana-Pérez, J.C.; Guevara-Salazar, J.A.; Trujillo-Ferrara, J.G.; Del Valle-Mondragón, L.; Arellano-Mendoza, M.G. Apocynin Combined with Drugs as Coadjuvant Could Be Employed to Prevent and/or Treat the Chronic Kidney Disease. Ren. Fail. 2018, 40, 92–98. [Google Scholar] [CrossRef]
- Boshtam, M.; Kouhpayeh, S.; Amini, F.; Azizi, Y.; Najaflu, M.; Shariati, L.; Khanahmad, H. Anti-Inflammatory Effects of Apocynin: A Narrative Review of the Evidence. Life 2021, 14, 997–1010. [Google Scholar] [CrossRef]
- Abdelmageed, M.E.; El-Awady, M.S.; Suddek, G.M. Apocynin Ameliorates Endotoxin-Induced Acute Lung Injury in Rats. Int. Immunopharmacol. 2016, 30, 163–170. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Song, Y.; Ye, X.; Fu, S.; Jiang, J.; Li, S. Improvement of Pharmacokinetics Behavior of Apocynin by Nitrone Derivatization: Comparative Pharmacokinetics of Nitrone-Apocynin and Its Parent Apocynin in Rats. PLoS ONE 2013, 8, e70189. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Suh, G.J.; Kwon, W.Y.; Kim, K.S.; Park, M.J.; Kim, T.; Ko, J.I. Apocynin Suppressed the Nuclear Factor-ΚB Pathway and Attenuated Lung Injury in a Rat Hemorrhagic Shock Model. J. Trauma Acute Care Surg. 2017, 82, 566–574. [Google Scholar] [CrossRef] [PubMed]
- ‘T Hart, B.A.; Simons, J.M.; Shoshan, K.-S.; Bakker, N.P.M.; Labadie, R.P. Antiarthritic Activity of the Newly Developed Neutrophil Oxidative Burst Antagonist Apocynin. Free Radic. Biol. Med. 1990, 9, 127–131. [Google Scholar] [CrossRef]
- ‘T Hart, B.A.; Copray, S.; Philippens, I. Apocynin, a Low Molecular Oral Treatment for Neurodegenerative Disease. BioMed Res. Int. 2014, 2014, 298020. [Google Scholar] [CrossRef] [PubMed]
- Van den Worm, E.; Beukelman, C.J.; Van den Berg, A.J.J.; Kroes, B.H.; Labadie, R.P.; Van Dijk, H. Effects of Methoxylation of Apocynin and Analogs on the Inhibition of Reactive Oxygen Species Production by Stimulated Human Neutrophils. Eur. J. Pharmacol. 2001, 433, 225–230. [Google Scholar] [CrossRef]
- Palmen, M.; Beukelman, C.; Mooij, R.; Pena, A.; Vonrees, E. Anti-Inflammatory Effect of Apocynin, a Plant-Derived NADPH Oxidase Antagonist, in Acute Experimental Colitis. Neth. J. Med. 1995, 47, A41. [Google Scholar] [CrossRef]
- Pandey, A.; Kour, K.; Bani, S.; Suri, K.A.; Satti, N.K.; Sharma, P.; Qazi, G.N. Amelioration of Adjuvant Induced Arthritis by Apocynin: Amelioration of adjuvant induced arthritis by apocynin. Phytother. Res. 2009, 23, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Harraz, M.M.; Marden, J.J.; Zhou, W.; Zhang, Y.; Williams, A.; Sharov, V.S.; Nelson, K.; Luo, M.; Paulson, H.; Schöneich, C.; et al. SOD1 Mutations Disrupt Redox-Sensitive Rac Regulation of NADPH Oxidase in a Familial ALS Model. J. Clin. Investig. 2008, 118, JCI34060. [Google Scholar] [CrossRef] [Green Version]
- Komogortsev, A.N.; Lichitsky, B.V.; Melekhina, V.G. Straightforward One-Step Approach towards Novel Derivatives of 9-Oxo-5,6,7,9-Tetrahydrobenzo[9,10]Heptaleno[3,2-b]Furan-12-Yl)Acetic Acid Based on the Multicomponent Reaction of Colchiceine, Arylglyoxals and Meldrum’s Acid. Tetrahedron Lett. 2021, 78, 153292. [Google Scholar] [CrossRef]
- Gorbunov, Y.O.; Lichitsky, B.V.; Komogortsev, A.N.; Mityanov, V.S.; Dudinov, A.A.; Krayushkin, M.M. Synthesis of Condensed Furylacetic Acids Based on Multicomponent Condensation of Heterocyclic Enols with Arylglyoxals and Meldrum’s Acid. Chem. Heterocycl. Compd. 2018, 54, 692–695. [Google Scholar] [CrossRef]
- Komogortsev, A.N.; Lichitsky, B.V.; Tretyakov, A.D.; Dudinov, A.A.; Krayushkin, M.M. Investigation of the Multicomponent Reaction of 5-Hydroxy-2-Methyl-4H-Pyran-4-One with Carbonyl Compounds and Meldrum’s Acid. Chem. Heterocycl. Compd. 2019, 55, 818–822. [Google Scholar] [CrossRef]
- Lichitsky, B.V.; Melekhina, V.G.; Komogortsev, A.N.; Minyaev, M.E. A New Multicomponent Approach to the Synthesis of Substituted Furan-2(5H)-Ones Containing 4H-Chromen-4-One Fragment. Tetrahedron Lett. 2020, 61, 152602. [Google Scholar] [CrossRef]
- Lichitsky, B.V.; Tretyakov, A.D.; Komogortsev, A.N.; Mityanov, V.S.; Dudinov, A.A.; Gorbunov, Y.O.; Daeva, E.D.; Krayushkin, M.M. Synthesis of Substituted Benzofuran-3-Ylacetic Acids Based on Three-Component Condensation of Polyalkoxyphenols, Arylglyoxals and Meldrum’s Acid. Mendeleev Commun. 2019, 29, 587–588. [Google Scholar] [CrossRef]
- Lichitsky, B.V.; Komogortsev, A.N.; Melekhina, V.G. 2-(2-(4-Methoxyphenyl)Furo[3,2-h]Quinolin-3-Yl)Acetic Acid. Molbank 2022, 2022, M1315. [Google Scholar] [CrossRef]
- Lichitsky, B.V.; Komogortsev, A.N.; Melekhina, V.G. 2-(2-(4-Methoxyphenyl)-4,9-Dimethyl-7-Oxo-7H-Furo[2,3-f]Chromen-3-Yl)Acetic Acid. Molbank 2021, 2021, M1304. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).