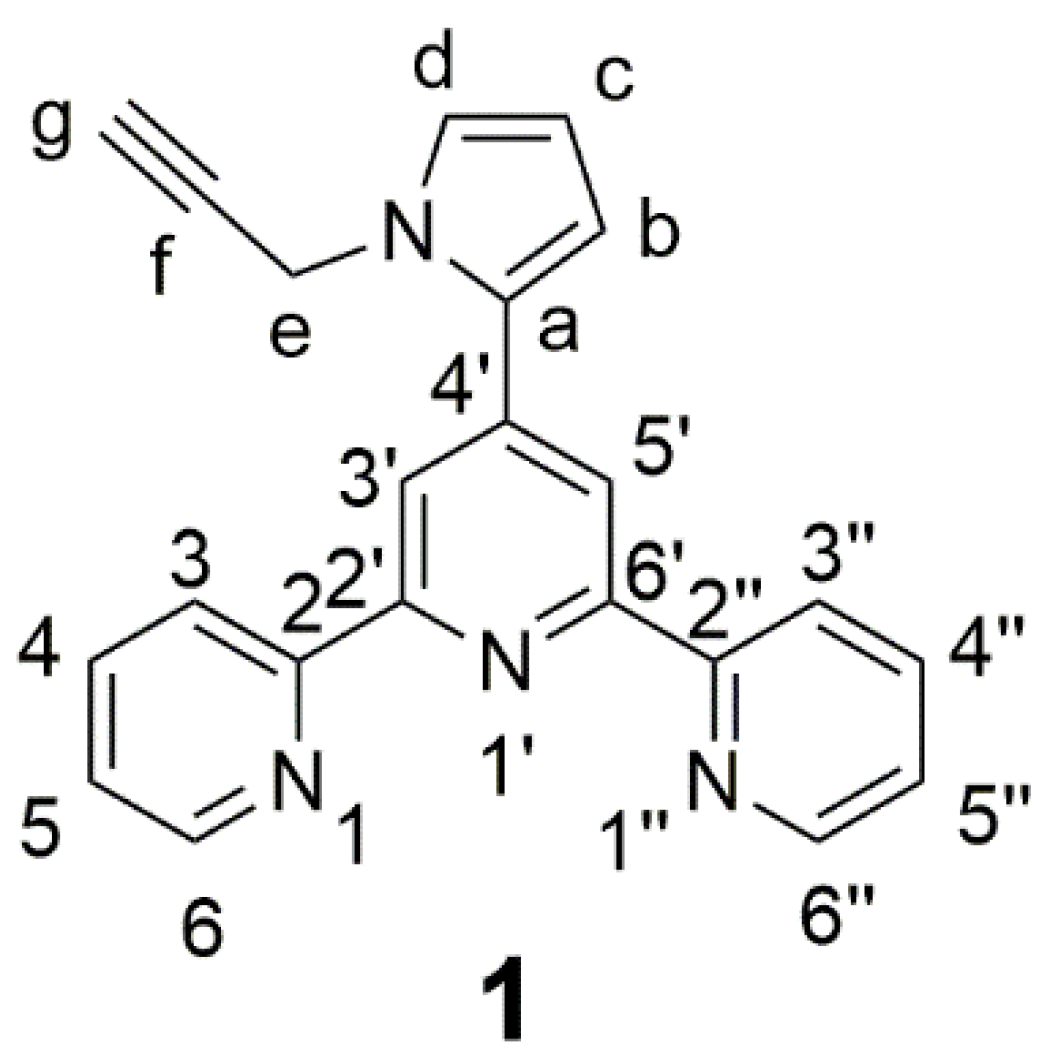
4′-(N-(Propargyl)pyrrol-2-yl)-2,2′:6′,2′′-terpyridine
Abstract
:1. Introduction
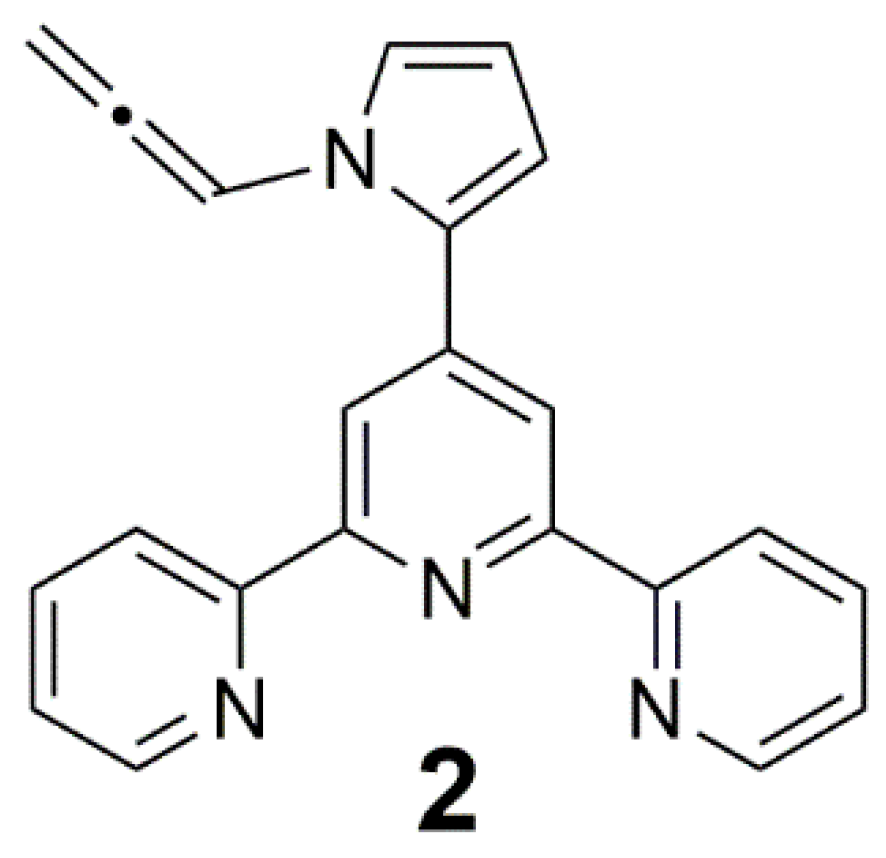
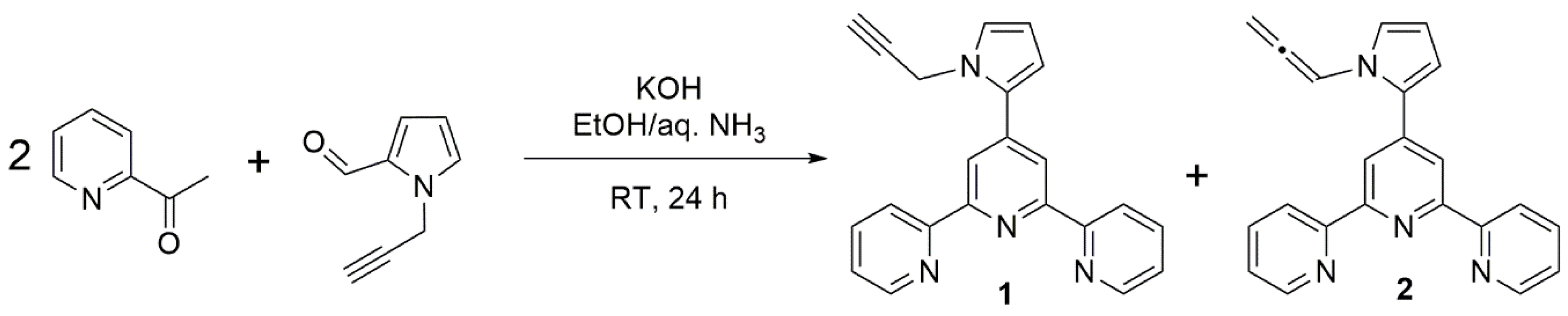
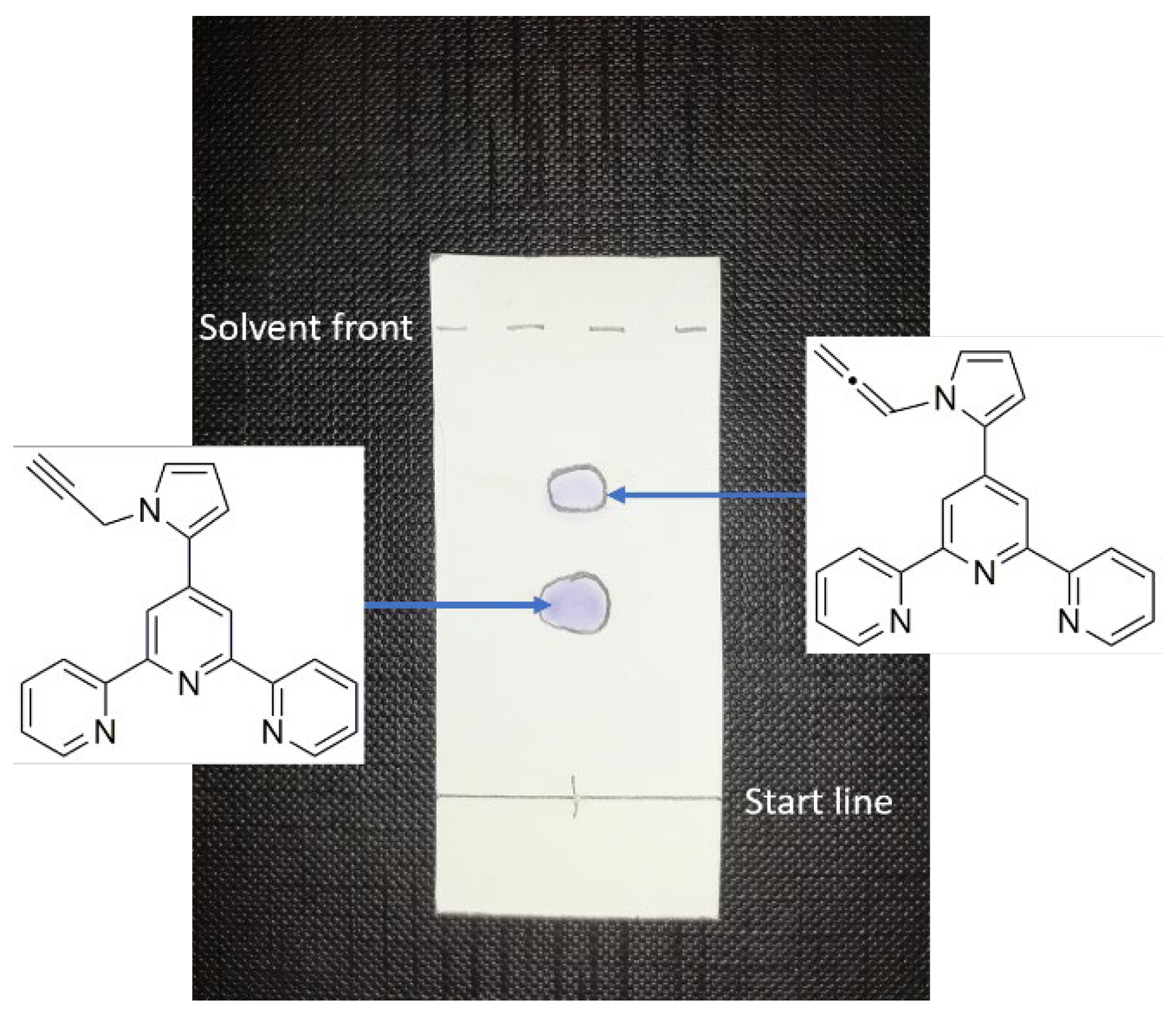
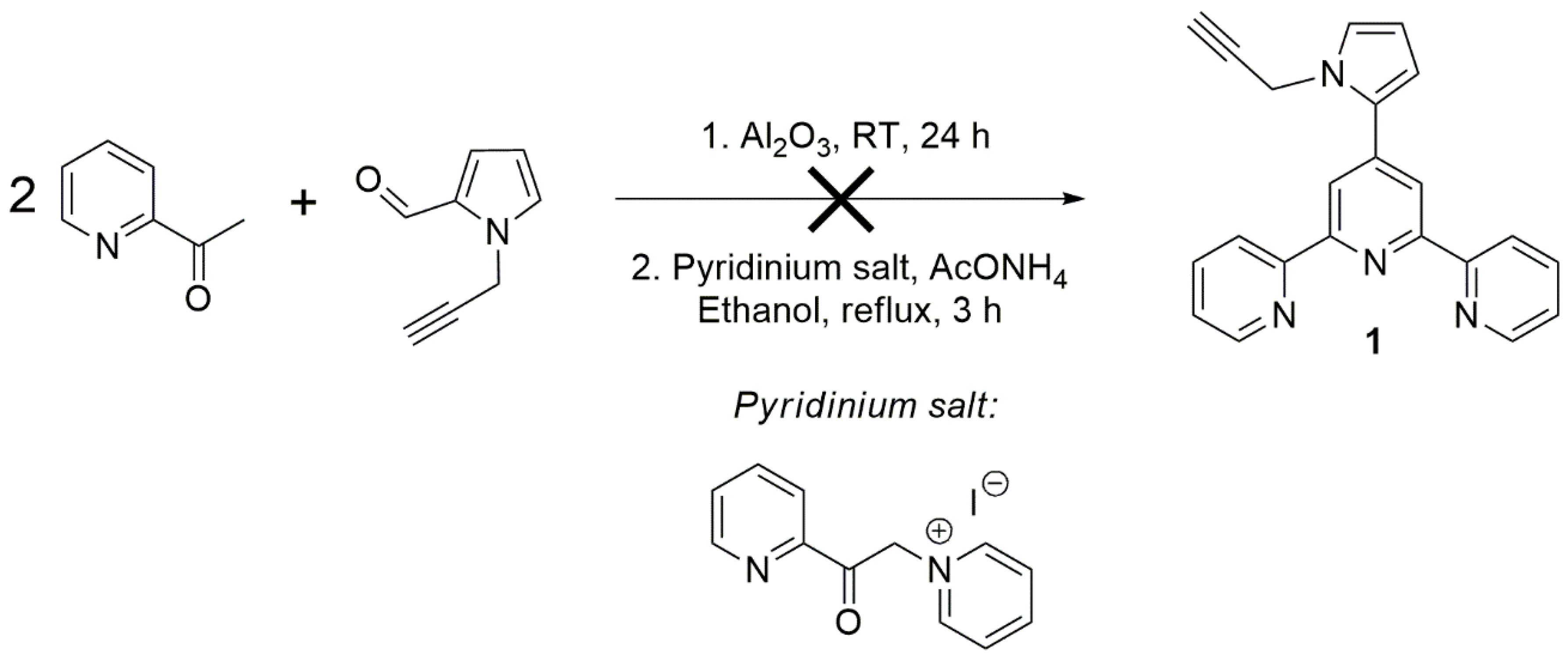
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Schubert, U.S.; Winter, A.; Newkome, G.R. Terpyridine-Based Materials: For Catalytic, Optoelectronic and Life Sciences Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Beley, M.; Delabouglise, D.; Houppy, G.; Husson, J.; Petit, J.-P. Preparation and properties of ruthenium (II) complexes of 2,2′:6′,2′′-terpyridines substituted at the 4′-position with heterocyclic groups. Inorg. Chim. Acta 2005, 358, 3075–3083. [Google Scholar] [CrossRef]
- Naidji, B.; Husson, J.; Taouil, A.E.; Brunol, E.; Sanchez, J.-B.; Berger, F.; Rauch, J.-Y.; Guyard, L. Terpyridine-based metallopolymer thin films as active layer in ammonia sensor device. Synth. Met. 2016, 221, 214–219. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. A new and facile method for the functionalization of a Merrifield resin with terpyridines: Application as a heterogeneous catalyst for the synthesis of biaryls in environmentally friendly solvents. Green Process. Synth. 2016, 5, 331–336. [Google Scholar] [CrossRef]
- Abboud, M.; Kalinina, D.; Potvin, P.G. Pyrrole-substituted tridentate complexes of Ru(II): Spectroscopy, electrochemistry, photosensitization and the role of orbital mixing. Inorg. Chim. Acta 2009, 362, 4953–4959. [Google Scholar] [CrossRef]
- Dehaudt, J.; Husson, J.; Guyard, L.; Oswald, F.; Martineau, D. A simple access to “Black-Dye” analogs with good efficiencies in dye-sensitized solar cells. Renew. Energy 2014, 66, 588–595. [Google Scholar] [CrossRef]
- Nakazawa, H.; Kobayashi, K. Base Metal-terpyridine Complex Immobilized on Stationary Phase Aimed as Reusable Hydrosilylation Catalyst. Chem. Asian J. 2021, 16, 3695–3701. [Google Scholar]
- Vilà, N.; Walcarius, A. Bis(terpyridine) Iron(II) Functionalized Vertically-Oriented Nanostructured Silica Films: Toward Electrochromic Materials. Front. Chem. 2021, 8, 830. [Google Scholar] [CrossRef]
- Busemann, A.; Araman, C.; Flaspohler, I.; Pratesi, A.; Zhou, X.-Q.; van Rixel, V.H.S.; Siegler, M.A.; Messori, L.; van Kasteren, S.I.; Bonnet, S. Alkyne Functionalization of a Photoactivated Polypyridyl Complex for Click-Enabled Serum Albumin interaction Studies. Inorg. Chem. 2020, 59, 7710–7720. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ Chemistry in Polymer and Material Science: An Update. Macromol. Rapid Commun. 2008, 29, 952–981. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Johnston, L.A.; Armspach, D.; Neuburger, M.; Zehnder, M. Dicobalt cluster functionalized 2,2′:6′,2′′-terpyridine ligands and their ruthenium(II) complexes. Polyhedron 2001, 20, 483–492. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. 4′-(N-(propan-1,2-dienyl)pyrrol-2-yl)-2,2′:6′,2′′-terpyridine. Molbank 2020, 2020, M1142. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. Synthesis of new 4′-(N-alkylpyrrol-2-yl)-2,2′:6′,2′′-terpyridines via N-alkylation of a pyrrole moiety. Heterocycl. Commun. 2015, 21, 199–202. [Google Scholar] [CrossRef]
- Yuan, Y.; Thome, I.; Kim, S.H.; Chen, D.T.; Beyer, A.; Bonnamour, J.; Zuidema, E.; Chang, S.; Bolm, C. Dimethyl Sulfoxide/Potassium Hydroxide: A Superbase for the Transition Metal-Free Preparation of Cross-Coupling Products. Adv. Synth. Catal. 2010, 17, 2892–2898. [Google Scholar] [CrossRef]
- Heller, M.; Schubert, U.S. Syntheses of functionalized 2,2′:6′,2′′-terpyridines. Eur. J. Org. Chem. 2003, 6, 947–961. [Google Scholar] [CrossRef]
- Fallahpour, R.A. Synthesis of 4′-substituted-2,2′:6′,2′′-terpyridines. Synthesis 2003, 2, 155–184. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The synthesis of 2,2′:6′,2′′-terpyridine ligands—Versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Kröhnke, F. Synthesis using pyridinium salts.5.Specific synthesis of pyridines and oligopyridines. Synthesis 1976, 1, 1–24. [Google Scholar] [CrossRef]
- Loaiza, P.R.; Löber, S.; Hübner, H.; Gmeiner, P. Click chemistry based solid phase supported synthesis of dopaminergic phenylacetylenes. Bioorg. Med. Chem. 2007, 15, 7248–7257. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-Hindered 4′-Aryl-2,2′:6′,2′′-Terpyridines. Synlett 2005, 8, 1251–1254. [Google Scholar] [CrossRef]
- Klemens, T.; Switlicka-Olszewska, A.; Machura, B.; Grucela, M.; Schab-Balcerzak, E.; Smolarek, K.; Mackowski, S.; Szlapa, A.; Kula, S.; Krompiec, S.; et al. Rhenium(I) terpyridine complexes—Synthesis, photophysical properties and application in organic light emitting devices. Dalton Trans. 2016, 45, 1746–1762. [Google Scholar] [CrossRef]
- Maron, A.; Szlapa, A.; Klemens, T.; Kula, S.; Machura, B.; Krompiec, S.; Malecki, J.G.; Switlicka-Olszewska, A.; Erfurt, K.; Chrobok, A. Tuning the photophysical properties of 4′-substituted terpyridines—An experimental and theoretical study. Org. Biomol. Chem. 2016, 14, 3793–3808. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, L.; Tarasova, O.A.; Kalinina, N.A.; Albanov, A.I.; Klyba, L.V.; Trofimov, B.A. Efficient Synthesis of 1-(2-Propynyl)pyrrole. Russ. J. Organ. Chem. 2002, 38, 1073–1075. [Google Scholar] [CrossRef]
- Das Adhikary, N.; Kwon, S.; Chung, W.-J.; Koo, S. One-Pot Conversion of Carbohydrates into Pyrrole-2-carbaldehydes as Sustainable Platform Chemicals. J. Org. Chem. 2015, 80, 7693–7701. [Google Scholar] [CrossRef] [PubMed]
- Husson, J.; Migianu, E.; Beley, M.; Kirsch, G. Synthesis of New Terpyridines under Solventless Conditions Using Alumina. Synthesis 2004, 2, 267–270. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husson, J.; Guyard, L. 4′-(N-(Propargyl)pyrrol-2-yl)-2,2′:6′,2′′-terpyridine. Molbank 2022, 2022, M1356. https://doi.org/10.3390/M1356
Husson J, Guyard L. 4′-(N-(Propargyl)pyrrol-2-yl)-2,2′:6′,2′′-terpyridine. Molbank. 2022; 2022(2):M1356. https://doi.org/10.3390/M1356
Chicago/Turabian StyleHusson, Jérôme, and Laurent Guyard. 2022. "4′-(N-(Propargyl)pyrrol-2-yl)-2,2′:6′,2′′-terpyridine" Molbank 2022, no. 2: M1356. https://doi.org/10.3390/M1356
APA StyleHusson, J., & Guyard, L. (2022). 4′-(N-(Propargyl)pyrrol-2-yl)-2,2′:6′,2′′-terpyridine. Molbank, 2022(2), M1356. https://doi.org/10.3390/M1356





