6-(2′-(4″-Oxabutyloxy)phenyl)-1,6,11-triaza-3,9,14,17,22,25-hexaoxa-2(1,2)(4-methylbenzena)-10(1,2)(5-methylbenzena)bicyclo(9.8.8)heptacosaphane Sodium Bromide Dichloromethane
Abstract
:1. Introduction
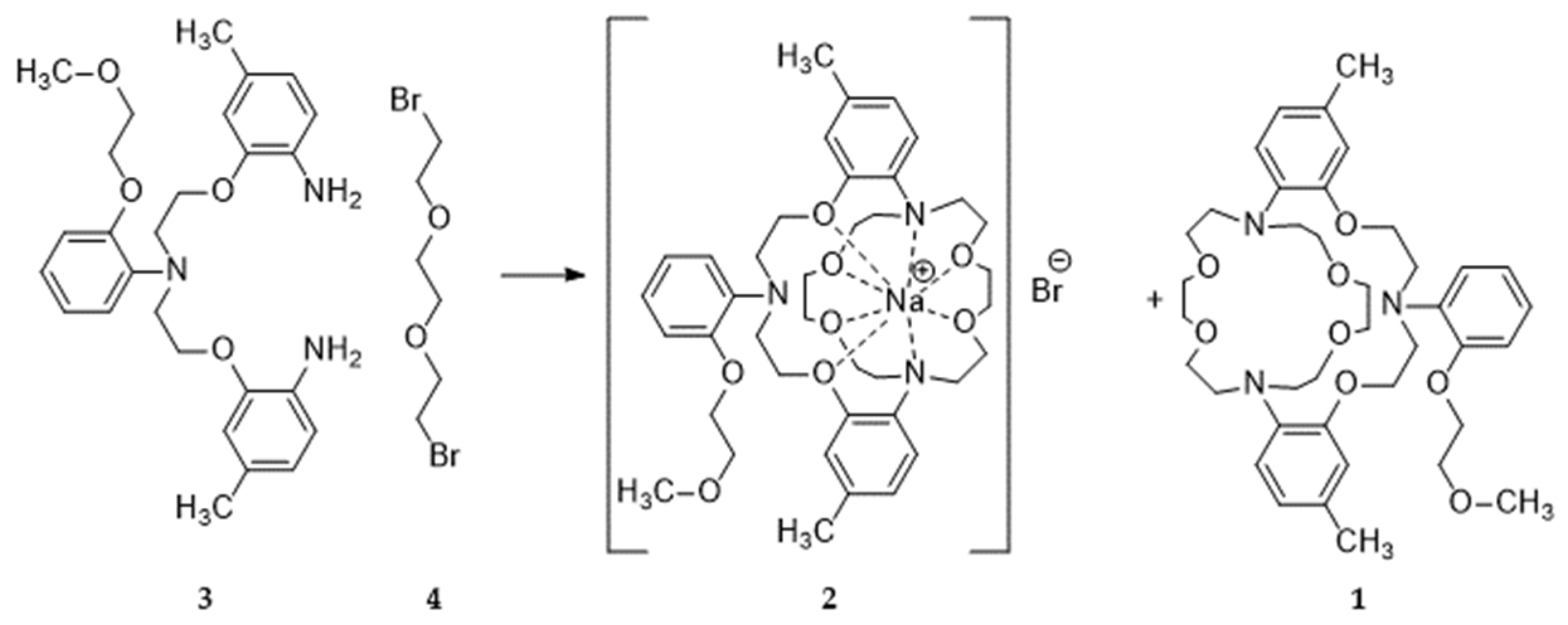
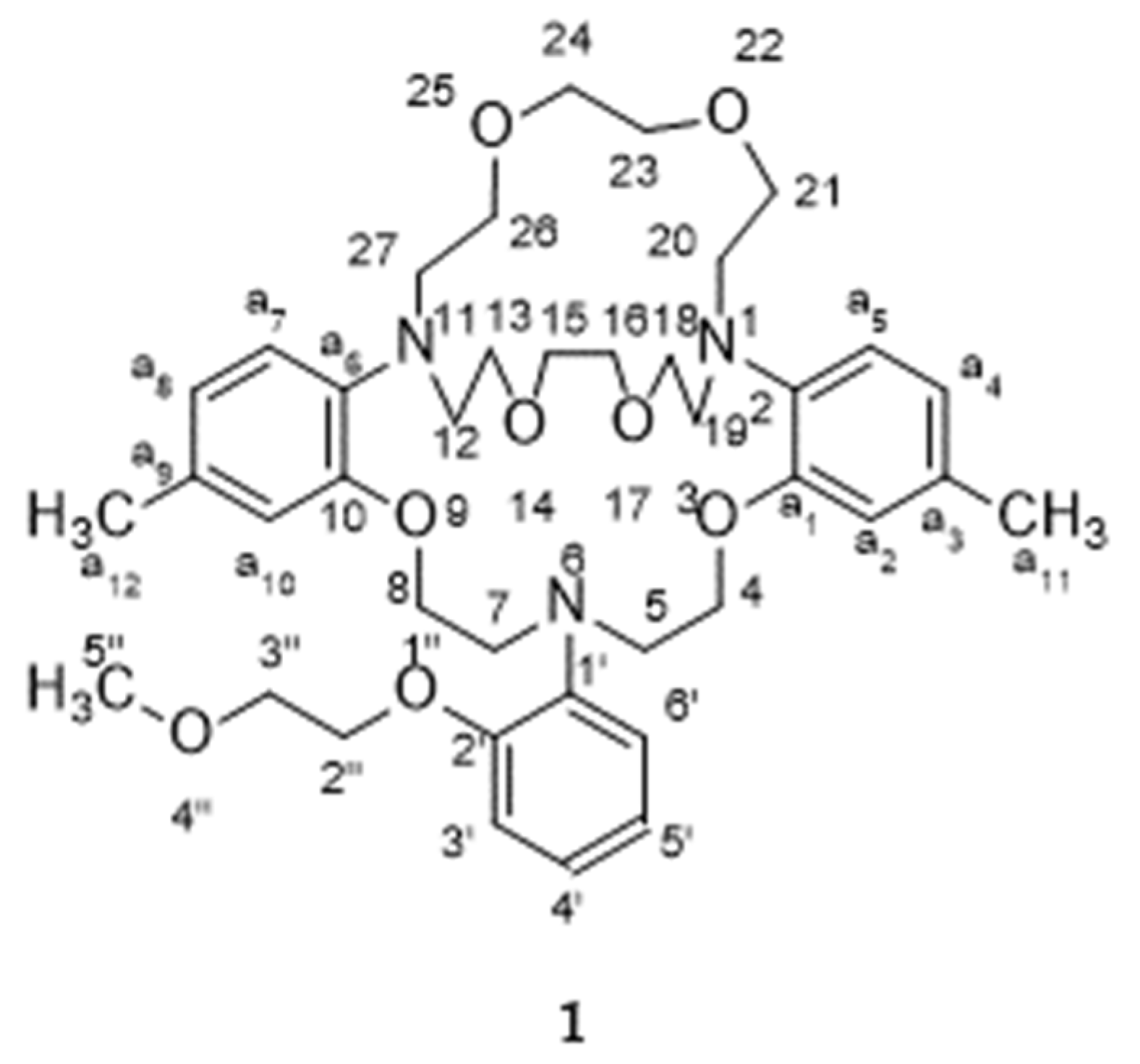
2. Results
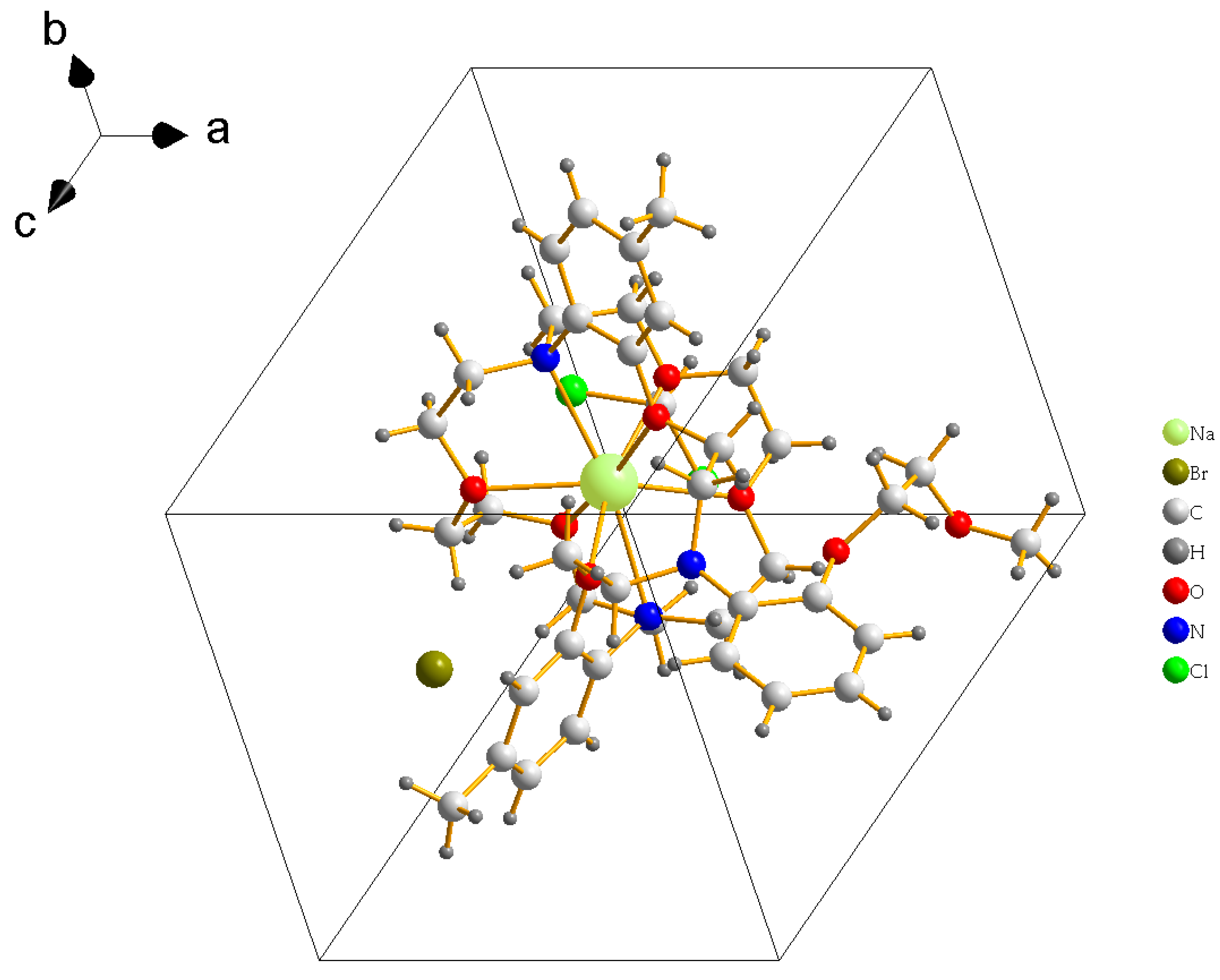
3. Crystal Structure
4. Discussion
5. Conclusions
6. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmer, B.F. Regulation of Potassium Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1050–1060. [Google Scholar] [CrossRef] [Green Version]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Keyster, M.; Niekerk, L.A.; Basson, G.; Carelse, M.; Bakare, O.; Ludidi, N.; Klein, A.; Mekuto, L.; Gokul, A. Decoding Heavy Metal Stress Signalling in Plants: Towards Improved Food Security and Safety. Plants 2020, 9, 1781. [Google Scholar] [CrossRef] [PubMed]
- Högberg, S.A.G.; Cram, D.J. Benzocrown Amino Ethers. J. Org. Chem. 1975, 40, 151–152. [Google Scholar] [CrossRef]
- He, H.; Martello, M.A.; Leiner, M.J.P.; Fraatz, R.J.; Tusa, J.K. A Fluorescent Sensor with High Selectivity and Sensitivity for Potassium in Water. J. Am. Chem. Soc. 2003, 125, 1468–1469. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chao, L.; Neeta, R.; Chuseng, L. Chromoionophore and Method of Determining Potassium Ions. U.S. Patent 7,960,181, 14 June 2011. [Google Scholar]
- Ruckh, T.T.; Skipwith, C.G.; Chang, W.; Senko, A.W.; Bulovic, V.; Anikeeva, P.O.; Clark, H.A. Ion-Switchable Quantum Dot Förster Resonance Energy Transfer Rates in Ratiometric Potassium Sensors. ACS Nano 2016, 10, 4020–4030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depauw, A.; Dossi, E.; Kumar, N.; Fiorini-Debuisschert, C.; Huberfeld, G.; Ha-Thi, M.-H.; Rouach, N.; Leray, I. A Highly Selective Potassium Sensor for the Detection of Potassium in Living Tissues. Chem. Eur. J. 2016, 22, 14902–14911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, R.D.; Verkman, A.S. Function-Oriented Synthesis of a Didesmethyl Triazacryptand Analogue for Fluorescent Potassium Ion Sensing. Eur. J. Org. Chem. 2011, 2011, 1242–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhammika Bandara, H.M.; Hua, Z.; Zhang, M.; Pauff, S.M.; Miller, S.C.; Colby Davie, E.A.; Kobertz, W.R. Palladium-Mediated Synthesis of a Near-Infrared Fluorescent K+ Sensor. J. Org. Chem. 2017, 82, 8199–8205. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.D.; Verkman, A.S. Synthesis of a Sensitive and Selective Potassium-Sensing Fluoroionophore. Org. Lett. 2010, 12, 116–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Su, F.; Tian, Y.; Youngbull, C.; Johnson, R.H.; Meldrum, D.R. A New Highly Selective Fluorescent K+ Sensor. J. Am. Chem. Soc. 2011, 133, 18530–18533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Su, F.; Gao, W.; Tian, Y.; Youngbull, C.; Johnson, R.H.; Meldrum, D.R. Triazacryptand-based fluorescent sensors for extracellular and intracellular K+ sensing. Biomaterials 2011, 32, 8574–8583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, B.; Yue, X.; Tichy, M.G.; Liu, T.; Belfield, K.D. Improved Synthesis of the Triazacryptand (TAC) and its Application in the Construction of a Fluorescent TAC-BODIPY Conjugate for K+ Sensing in Live Cells. Eur. J. Org. Chem. 2015, 2015, 1189–1192. [Google Scholar] [CrossRef]
- Vögtle, F.; Weber, E. Crown ethers—Complexes and selectivity. In The Chemistry of Ethers, Crown Ethers, Hydroxyl Groups and Their Sulphur Analogues; Patai, S., Ed.; Wiley: Hoboken, NJ, USA, 1980; Volume 1, pp. 59–156. [Google Scholar]
- Kim, J.; Shamsipur, M.; Huang, S.Z.; Huang, R.H.; Dye, J.L. Sandwich and Mixed Sandwich Complexes of the Cesium Ion with Crown Ethers in Nitromethane. J. Phys. Chem. A 1999, 103, 5615–5620. [Google Scholar] [CrossRef]
- Dapporto, P.; Paoli, P.; Matijasic, I.; Tusek-Bozic, L. Synthesis, characterization and crystal structures of complexes of sodium hexafluorophosphate with dibenzo-18-crown-6 and dibenzo-24-crown-8 macrocycle. Inorg. Chim. Acta 1998, 282, 76–81. [Google Scholar] [CrossRef]
- CCDC 2130482 (2) Contains the Supplementary Crystallographic Data for this Paper. These Data can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/datarequest/cif (accessed on 21 December 2021).
- Steed, J.W. First and second-sphere coordination chemistry of alkali metal crown ether complexes. Coord. Chem. Rev. 2001, 215, 171–221. [Google Scholar] [CrossRef]
- Dalley, N.K.; Krakowiak, K.E.; Bradshaw, J.S.; England, M.M.; Kou, X.; Izatt, R.M. Crystal Structure of the Cryptand [3.3.3]—Potassium Iodide Complex: A large Coordination Number for Potassium Ion. Tetrahedron 1994, 50, 2721–2728. [Google Scholar] [CrossRef]
- Bechtolsheimer, H.-H.; Buchholz, M.; Kunz, H. Die Reaktion von 2-Halogenethoxycarbonyl-Verbindungen mit tertiären Phosphanen. Liebigs Ann. Chem. 1979, 11, 1908–1914. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jochem, M.; Schollmeyer, D.; Detert, H. 6-(2′-(4″-Oxabutyloxy)phenyl)-1,6,11-triaza-3,9,14,17,22,25-hexaoxa-2(1,2)(4-methylbenzena)-10(1,2)(5-methylbenzena)bicyclo(9.8.8)heptacosaphane Sodium Bromide Dichloromethane. Molbank 2022, 2022, M1348. https://doi.org/10.3390/M1348
Jochem M, Schollmeyer D, Detert H. 6-(2′-(4″-Oxabutyloxy)phenyl)-1,6,11-triaza-3,9,14,17,22,25-hexaoxa-2(1,2)(4-methylbenzena)-10(1,2)(5-methylbenzena)bicyclo(9.8.8)heptacosaphane Sodium Bromide Dichloromethane. Molbank. 2022; 2022(1):M1348. https://doi.org/10.3390/M1348
Chicago/Turabian StyleJochem, Matthias, Dieter Schollmeyer, and Heiner Detert. 2022. "6-(2′-(4″-Oxabutyloxy)phenyl)-1,6,11-triaza-3,9,14,17,22,25-hexaoxa-2(1,2)(4-methylbenzena)-10(1,2)(5-methylbenzena)bicyclo(9.8.8)heptacosaphane Sodium Bromide Dichloromethane" Molbank 2022, no. 1: M1348. https://doi.org/10.3390/M1348
APA StyleJochem, M., Schollmeyer, D., & Detert, H. (2022). 6-(2′-(4″-Oxabutyloxy)phenyl)-1,6,11-triaza-3,9,14,17,22,25-hexaoxa-2(1,2)(4-methylbenzena)-10(1,2)(5-methylbenzena)bicyclo(9.8.8)heptacosaphane Sodium Bromide Dichloromethane. Molbank, 2022(1), M1348. https://doi.org/10.3390/M1348






