O-((Ferrocenyl)(3-fluorophenyl)methyl)hydroxylamine
Abstract
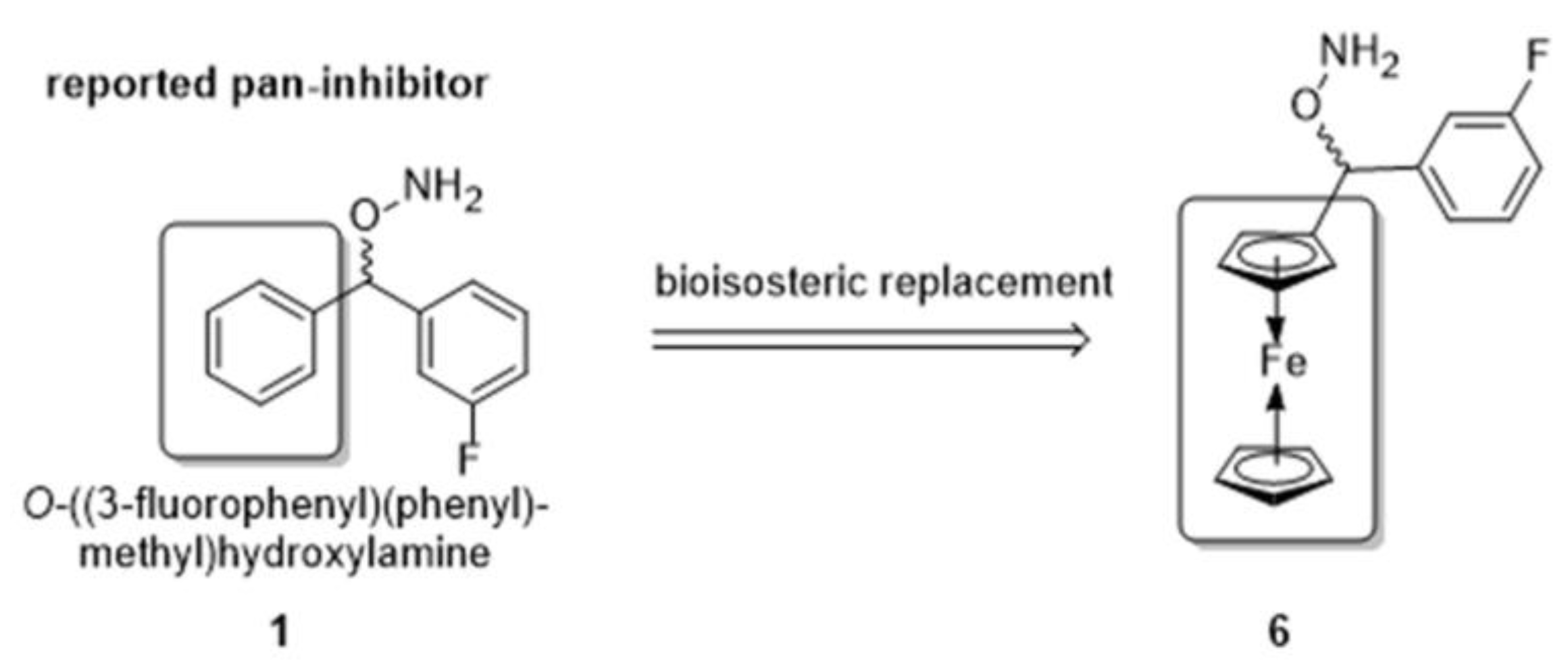
:1. Introduction
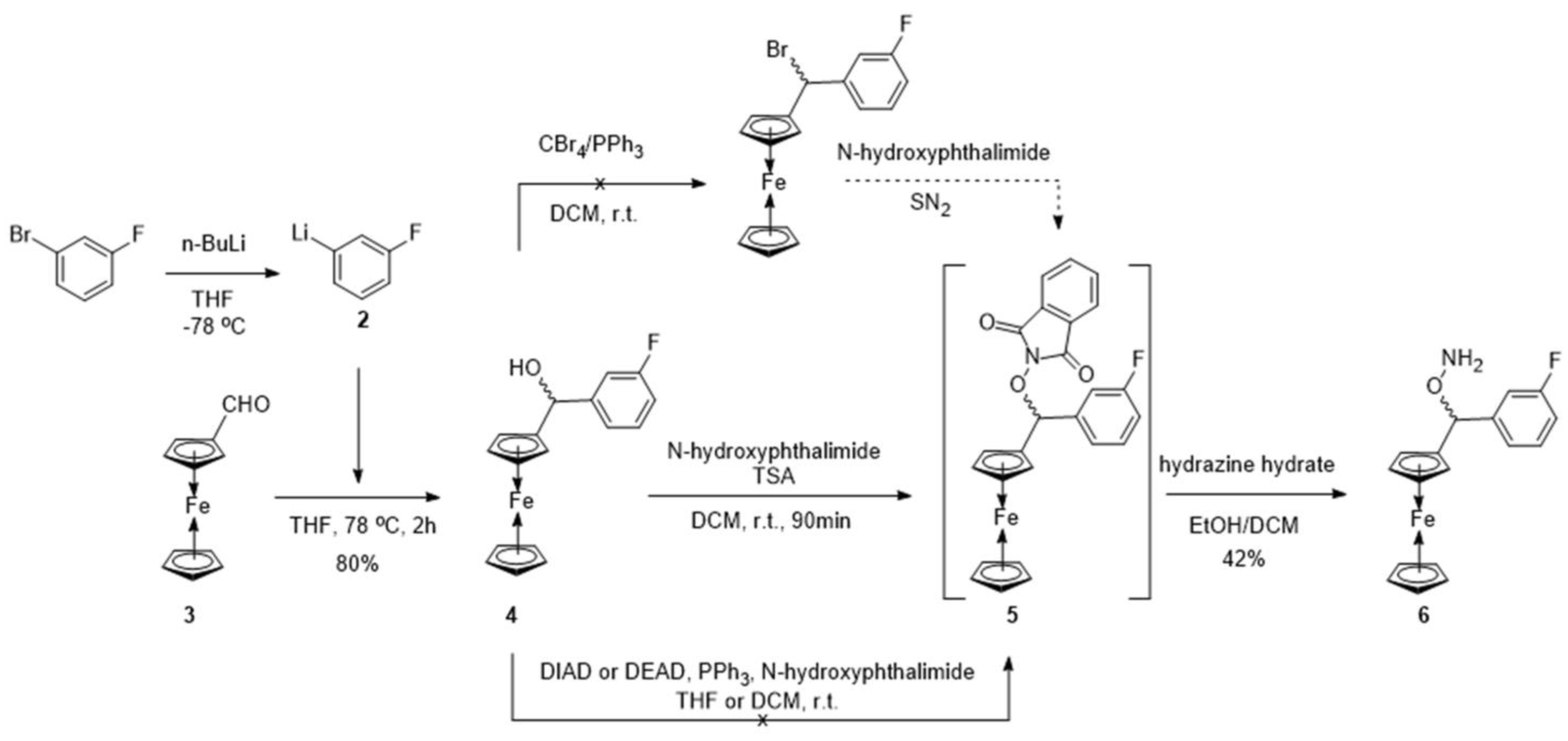
2. Results and Discussion
3. Materials and Methods
3.1. General
3.1.1. (Ferrocenyl)(3-fluorophenyl)methanol
3.1.2. 2-((Ferrocenyl)(3-fluorophenyl)methoxy)isoindoline-1,3-dione
3.1.3. O-((Ferrocenyl)(3-fluorophenyl)methyl)hydroxylamine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-appended pharmacophores: An exciting approach for modulating the biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpin, K.J.; Dyson, P.J. Enzyme inhibition by metal complexes: Concepts, strategies and applications. Chem. Sci. 2013, 4, 1410–1419. [Google Scholar] [CrossRef] [Green Version]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Lewis-Ballester, A.; Pham, K.N.; Batabyal, D.; Karkashon, S.; Bonanno, J.B.; Poulos, T.L.; Yeh, S.R. Structural insights into substrate and inhibitor binding sites in human indoleamine 2,3-dioxygenase. 1. Nat. Commun. 2017, 8, 1693. [Google Scholar] [CrossRef] [PubMed]
- Winters, M.; DuHadaway, J.B.; Pham, K.N.; Lewis-Ballester, A.; Badir, S.; Wai, J.; Sheikh, E.; Yeh, S.R.; Prendergast, G.C.; Muller, A.J.; et al. Diaryl hydroxylamines as pan or dual inhibitors of indoleamine 2,3-dioxygenase-1, indoleamine 2,3-dioxygenase-2 and tryptophan dioxygenase. Eur. J. Med. Chem. 2019, 162, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Juric, S.; Marijan, M.; Kronja, O. Electrofugality of Some Ferrocenylphenylmethyl Cations. Croat. Chem. Acta 2019, 92, 307–313. [Google Scholar] [CrossRef]
- Marijan, M.; Juric, S.; Mihalic, Z.; Kronja, O. Impact of the alpha-Ferrocenyl Group on the Solvolytic Reactivity-Electrofugality-of Ferrocenylphenylmethyl Cations. Eur. J. Org. Chem. 2019, 2019, 537–546. [Google Scholar] [CrossRef]
- Reddy, C.R.; Radhika, L.; Kumar, T.P.; Chandrasekhar, S. First Acid-Catalyzed Entry to O-Alkylated Hydroximides from Benzylic Alcohols. Eur. J. Org. Chem. 2011, 2011, 5967–5970. [Google Scholar] [CrossRef]
- Lind, J.; Merenyi, G. Kinetic and thermodynamic properties of the aminoxyl (NH2O) radical. J. Phys. Chem. A 2006, 110, 192–197. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foscolos, A.S.; Georgiou, M.; Papadopoulos, M.S.; Chiotellis, A. O-((Ferrocenyl)(3-fluorophenyl)methyl)hydroxylamine. Molbank 2022, 2022, M1346. https://doi.org/10.3390/M1346
Foscolos AS, Georgiou M, Papadopoulos MS, Chiotellis A. O-((Ferrocenyl)(3-fluorophenyl)methyl)hydroxylamine. Molbank. 2022; 2022(1):M1346. https://doi.org/10.3390/M1346
Chicago/Turabian StyleFoscolos, Angeliki S., Maria Georgiou, Minas S. Papadopoulos, and Aristeidis Chiotellis. 2022. "O-((Ferrocenyl)(3-fluorophenyl)methyl)hydroxylamine" Molbank 2022, no. 1: M1346. https://doi.org/10.3390/M1346
APA StyleFoscolos, A. S., Georgiou, M., Papadopoulos, M. S., & Chiotellis, A. (2022). O-((Ferrocenyl)(3-fluorophenyl)methyl)hydroxylamine. Molbank, 2022(1), M1346. https://doi.org/10.3390/M1346






