Synthesis and Crystal Structure of 9,12-Dibromo-ortho-Carborane
Abstract
:1. Introduction
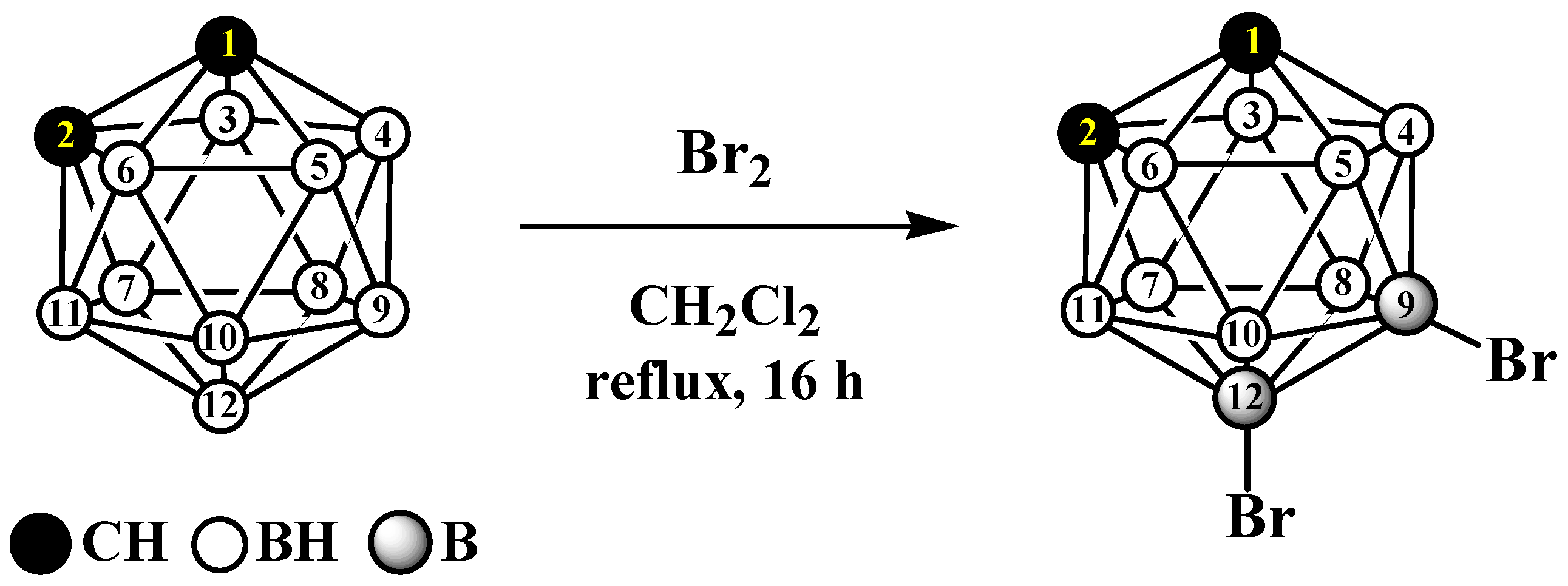
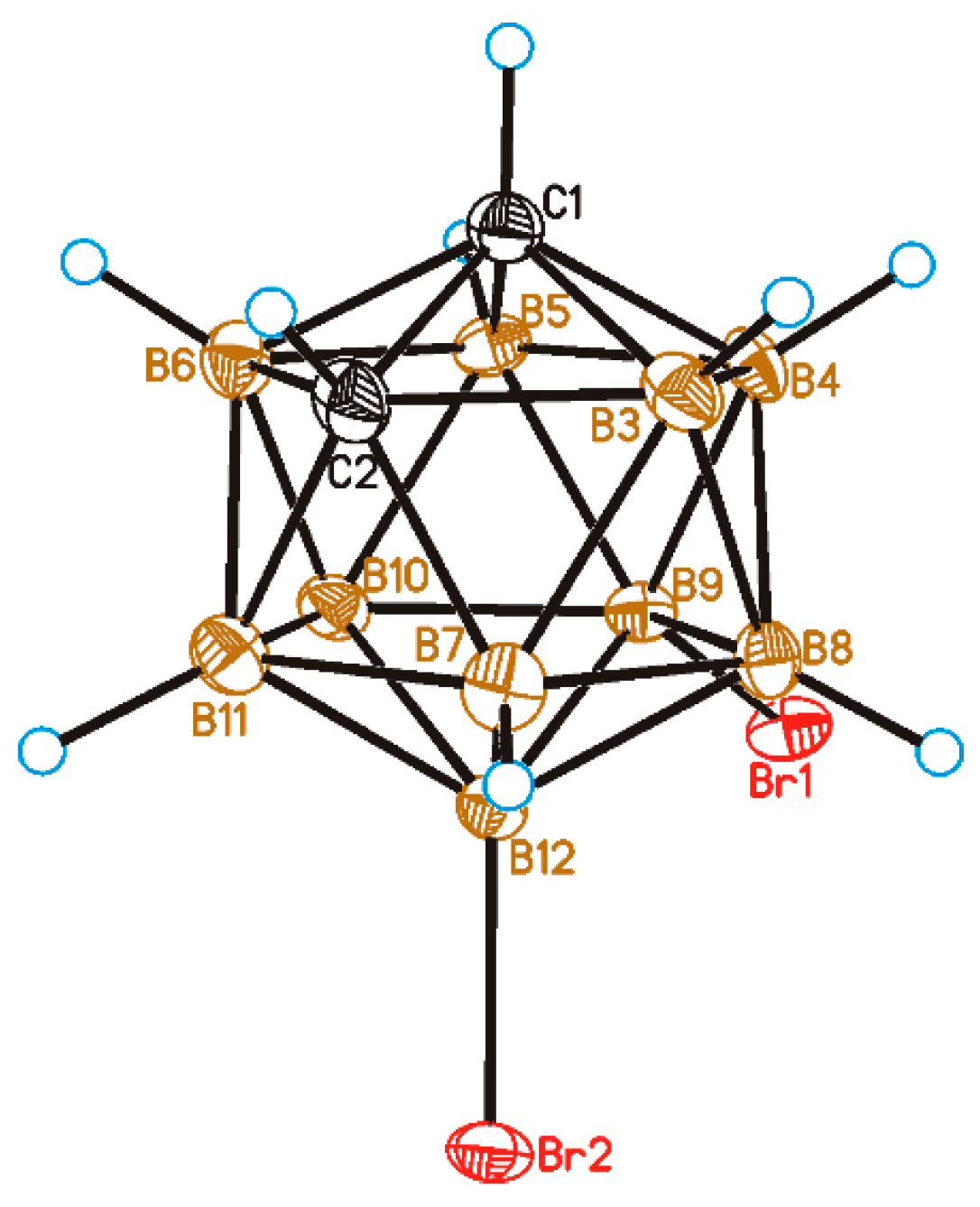
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valliant, J.F.; Guenther, K.J.; King, A.S.; Morel, P.; Schaffer, P.; Sogbein, O.O.; Stephenson, K.A. The medicinal chemistry of carboranes. Coord. Chem. Rev. 2002, 232, 173–230. [Google Scholar] [CrossRef]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Properties, synthesis, and application strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef] [PubMed]
- Leśnikowski, Z.J. Challenges and opportunities for the application of boron clusters in drug design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sárosi, M.B.; Hey-Hawkins, E. New keys for old locks: Carborane-containing drugs as platforms for mechanism-based therapies. Chem. Soc. Rev. 2019, 48, 3497–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, N.; McCarthy, E.; Dwyer, R.; Farràs, P. Boron clusters as breast cancer therapeutics. J. Inorg. Biochem. 2021, 218, 111412. [Google Scholar] [CrossRef]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coord. Chem. Rev. 2021, 431, 213684. [Google Scholar] [CrossRef]
- Gruzdev, D.A.; Levit, G.L.; Krasnov, V.P.; Charushin, V.N. Carborane-containing amino acids and peptides: Synthesis, properties and applications. Coord. Chem. Rev. 2021, 433, 213753. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Farha, O.K.; Mulfort, K.L.; Hupp, J.T.; Mirkin, C.A. Porosity tuning of carborane-based metal-organic frameworks (MOFs) via coordination chemistry and ligand design. Inorg. Chim. Acta 2010, 364, 266–271. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Krungleviciute, V.; Clingerman, D.J.; Mondloch, J.E.; Peng, Y.; Wilmer, C.; Sarjeant, A.A.; Snurr, R.; Hupp, J.T.; Yildirim, T.; et al. Carborane-based metal–organic framework with high methane and hydrogen storage capacities. Chem. Mater. 2013, 25, 3539–3543. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yan, H.; Zhao, Q. Carboranes as a tool to tune phosphorescence. Chem. Eur. J. 2015, 22, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Boron clusters in luminescent materials. Chem. Commun. 2015, 52, 1070–1093. [Google Scholar] [CrossRef] [PubMed]
- Ochi, J.; Tanaka, K.; Chujo, Y. Recent progress in the development of solid-state luminescent o-carboranes with stimuli responsivity. Angew. Chem. Int. Ed. 2020, 132, 9925–9939. [Google Scholar] [CrossRef]
- Yan, J.; Yang, W.; Zhang, Q.; Yan, Y. Introducing borane clusters into polymeric frameworks: Architecture, synthesis, and applications. Chem. Commun. 2020, 56, 11720–11734. [Google Scholar] [CrossRef]
- Aniés, F.; Qiao, Z.; Nugraha, M.I.; Basu, A.; Anthopoulos, T.D.; Gasparini, N.; Heeney, M. N-type polymer semiconductors incorporating para, meta, and ortho-carborane in the conjugated backbone. Polymer 2021, 240, 124481. [Google Scholar] [CrossRef]
- Pecyna, J.; Jankowiak, A.; Pociechac, D.; Kaszyński, P. o-Carborane derivatives for probing molecular polarity effects on liquid crystal phase stability and dielectric behavior. J. Mater. Chem. C 2015, 3, 11412–11422. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; González-Campo, A.; Planas, J.G.; Viñas, C.; Teixidor, F.; Sáez, I.M.; Núñez, R. Tuning the liquid crystallinity of cholesteryl-o-carborane dyads: Synthesis, structure, photoluminescence, and mesomorphic properties. Crystals 2021, 11, 133. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, J.; Chen, X.; Cheng, C.; Chu, D.; Tang, X.; Li, H.; Cui, Y. Synthesis, structure and property of boron-based metal-organic materials. Coord. Chem. Rev. 2021, 435, 213783. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: London, UK, 2016; pp. 283–502. [Google Scholar] [CrossRef]
- Andrews, J.S.; Zayas, J.; Jones, M. 9-Iodo-o-carborane. Inorg. Chem. 1985, 24, 3715–3716. [Google Scholar] [CrossRef]
- Li, J.; Logan, C.F.; Jones, M. Simple syntheses and alkylation reactions of 3-iodo-o-carborane and 9,12-diiodo-o-carborane. Inorg. Chem. 1991, 30, 4866–4868. [Google Scholar] [CrossRef]
- Zheng, Z.; Jiang, W.; Zinn, A.A.; Knobler, C.B.; Hawthorne, M.F. Facile electrophilic iodination of icosahedral carboranes. Synthesis of carborane derivatives with boron-carbon bonds via the palladium- catalyzed reaction of diiodocarboranes with Grignard reagents. Inorg. Chem. 1995, 34, 2095–2100. [Google Scholar] [CrossRef]
- Jiang, W.; Knobler, C.B.; Curtis, C.E.; Mortimer, M.D.; Hawthorne, M.F. Iodination reactions of icosahedral para-carborane and the synthesis of carborane derivatives with boron-carbon bonds. Inorg. Chem. 1995, 34, 3491–3498. [Google Scholar] [CrossRef]
- Barberà, G.; Teixidor, F.; Viñas, C.; Sillanpää, R.; Kivekäs, R. Sequential nucleophilic-electrophilic reactions selectively produce isomerically pure nona-B-substituted o-carborane derivatives. Eur. J. Inorg. Chem. 2003, 1511–1513. [Google Scholar] [CrossRef]
- Yamazaki, H.; Ohta, K.; Endo, Y. Regioselective synthesis of triiodo-o-carboranes and tetraiodo-o-carborane. Tetrahedron Lett. 2005, 46, 3119–3122. [Google Scholar] [CrossRef]
- Vaca, A.; Teixidor, F.; Kivekäs, R.; Sillanpää, R.; Viñas, C. A solvent-free regioselective iodination route of ortho-carboranes. Dalton Trans. 2006, 4884–4885. [Google Scholar] [CrossRef] [PubMed]
- Teixidor, F.; Barberà, G.; Viñas, C.; Sillanpää, R.; Kivekäs, R. Synthesis of boron-iodinated o-carborane derivatives. Water stability of the periodinated monoprotic salt. Inorg. Chem. 2006, 45, 3496–3498. [Google Scholar] [CrossRef] [PubMed]
- Barberà, G.; Vaca, A.; Teixidor, F.; Sillanpää, R.; Kivekäs, R.; Viñas, C. Designed synthesis of new ortho-carborane derivatives: From mono- to polysubstituted frameworks. Inorg. Chem. 2008, 47, 7309–7316. [Google Scholar] [CrossRef]
- Safronov, A.V.; Sevryugina, Y.V.; Jalisatgi, S.S.; Kennedy, R.D.; Barnes, C.L.; Hawthorne, M.F. Unfairly forgotten member of the iodocarborane family: Synthesis and structural characterization of 8-iodo-1,2-dicarba-closo-dodecaborane, its precursors, and derivatives. Inorg. Chem. 2012, 51, 2629–2637. [Google Scholar] [CrossRef]
- Lyu, H.; Quan, Y.; Xie, Z. Transition metal catalyzed, regioselective B(4)-halogenation and B(4,5)-diiodination of cage B-H bonds in o-carboranes. Chem. Eur. J. 2017, 23, 14866–14871. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Koveredov, A.I.; Ol’shevskaya, V.A.; Shaugumbekova, S. Synthesis of B-organo-substituted 1,2-, 1,7-, and 1,12-dicarba-closo-dodecarboranes(12). J. Organomet. Chem. 1982, 226, 217–226. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Balagurova, E.V.; Lebedev, V.N. Suzuki cross-coupling in the carborane series. Russ. J. Gen. Chem. 1998, 68, 922–924. [Google Scholar]
- Viñas, C.; Barberà, G.; Oliva, J.M.; Teixidor, F.; Welch, A.J.; Rosair, G.M. Are halocarboranes suitable for substitution reactions? The case for 3-I-1,2-closo-C2B10H11: Molecular orbital calculations, aryldehalogenation reactions, 11B NMR interpretation of closo-carboranes, and molecular structures of 1-Ph-3-Br-1,2-closo-C2B10H10 and 3-Ph-1,2-closo-C2B10H11. Inorg. Chem. 2001, 40, 6555–6562. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Beletskaya, I.P.; Bregadze, V.I.; Sivaev, I.B.; Sjöberg, S. Palladium-catalyzed cross-coupling reactions of arylboronic acids and 2-I-p-carborane. J. Organomet. Chem. 2002, 657, 267–272. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Bregadze, V.I.; Ivushkin, V.A.; Petrovskii, P.V.; Sivaev, I.B.; Sjöberg, S.; Zhigareva, G.G. New B-substituted derivatives of m-carborane, p-carborane, and cobalt bis(1,2-dicarbollide) anion. J. Organomet. Chem. 2004, 689, 2920–2929. [Google Scholar] [CrossRef]
- Aizawa, K.; Ohta, K.; Endo, Y. Synthesis of 3-aryl-1,2-dicarba-closo-dodecaboranes by Suzuki-Miyaura coupling reaction. Heterocycles 2010, 80, 369–377. [Google Scholar] [CrossRef]
- Jankowiak, A.; Kaszyński, P. Practical synthesis of 1,12-difunctionalized o-carborane for the investigation of polar liquid crystals. Inorg. Chem. 2014, 53, 8762–8769. [Google Scholar] [CrossRef]
- Anderson, K.P.; Mills, H.A.; Mao, C.; Kirlikovali, K.O.; Axtell, J.C.; Rheingold, A.L.; Spokoyny, A.M. Improved synthesis of icosahedral carboranes containing exopolyhedral B-C and C-C bonds. Tetrahedron 2018, 75, 187–191. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Shmal’ko, A.V.; Suponitsky, K.Y.; Sivaev, I.B. One-pot synthesis of B-aryl carboranes with sensitive functional groups using sequential cobalt- and palladium-catalyzed reactions. Catalysts 2020, 10, 1348. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Shmal’ko, A.V.; Suponitsky, K.Y.; Sivaev, I.B. Synthesis of 3-aryl-ortho-carboranes with sensitive functional groups. Molecules 2021, 26, 7297. [Google Scholar] [CrossRef]
- Puga, A.V.; Teixidor, F.; Sillanpää, R.; Kivekäs, R.; Viñas, C. Iodinated ortho-carboranes as versatile building blocks to design intermolecular interactions in crystal lattices. Chem. Eur. J. 2009, 15, 9764–9772. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Anisimov, A.A.; Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. 1,12-Diiodo-ortho-carborane: A classic textbook example of the dihalogen bond. Crystals 2020, 11, 396. [Google Scholar] [CrossRef]
- Ohta, K.; Ogawa, T.; Endo, Y. Design and synthesis of iodocarborane-containing ligands with high affinity and selectivity toward ERβ. Bioorg. Med. Chem. Lett. 2017, 27, 4030–4033. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.D.; Knowles, T.A.; Schroeder, H. Chemistry of decaborane-phosphorus Compounds. V. Bromocarboranes and their phosphination. Inorg. Chem. 1965, 4, 107–111. [Google Scholar] [CrossRef]
- Dziedzic, R.M.; Saleh, L.M.A.; Axtell, J.C.; Martin, J.L.; Stevens, S.L.; Royappa, A.T.; Rheingold, A.L.; Spokoyny, A.M. B-N, B-O, and B-CN bond formation via palladium-catalyzed cross-coupling of B-bromo-carboranes. J. Am. Chem. Soc. 2016, 138, 9081–9084. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, R.M.; Martin, J.L.; Axtell, J.C.; Saleh, L.M.A.; Ong, T.-C.; Yang, Y.-F.; Messina, M.S.; Rheingold, A.L.; Houk, K.N.; Spokoyny, A.M. Cage-walking: Vertex differentiation by palladium-catalyzed isomerization of B(9)-bromo-meta-carborane. J. Am. Chem. Soc. 2017, 139, 7729–7732. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Hopp, M.; Dziedzic, R.M.; Waddington, M.A.; Rheingold, A.L.; Sletten, E.M.; Axtell, J.C.; Spokoyny, A.M. Expanding the scope of palladium-catalyzed B-N cross-coupling chemistry in carboranes. Organometallics 2020, 39, 4380–4386. [Google Scholar] [CrossRef]
- Kataki-Anastasakou, A.; Axtell, J.C.; Hernandez, S.; Dziedzic, R.M.; Balaich, G.J.; Rheingold, A.L.; Spokoyny, A.M.; Sletten, E.M. Carborane guests for cucurbit[7]uril facilitate strong binding and on-demand removal. J. Am. Chem. Soc. 2020, 142, 20513–20518. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Holub, J.; Růžičková, Z.; Řezáč, J.; Lane, P.D.; Wann, D.A.; Hnyk, D.; Růžička, A.; Hobza, P. Competition between halogen, hydrogen and dihydrogen bonding in brominated carboranes. ChemPlusChem 2016, 17, 3373–3376. [Google Scholar] [CrossRef]
- Potenza, J.A.; Lipscomb, W.N. Molecular structure of carboranes. Molecular and crystal structure of o-B10Br2H8C2H2. Inorg. Chem. 1966, 5, 1471–1477. [Google Scholar] [CrossRef]
- Potenza, J.A.; Lipscomb, W.N. Molecular structure of carboranes. Molecular and crystal structure of o-B10Br3H7C2H2. Inorg. Chem. 1966, 5, 1478–1482. [Google Scholar] [CrossRef]
- Beall, H.A.; Lipscomb, W.N. Molecular and crystal structure of m-B10Br2H8C2H2. Inorg. Chem. 1967, 6, 874–879. [Google Scholar] [CrossRef]
- Sayler, A.A.; Beall, H. The crystal and molecular structure of tribromo-m-carborane. Can. J. Chem. 1976, 54, 1771–1776. [Google Scholar] [CrossRef]
- Potenza, J.A.; Lipscomb, W.N.; Vickers, G.D.; Schroeder, H. Order of electrophilic substitution in 1,2-dicarbaclovododecaborane(l2) and nuclear magnetic resonance assignment. J. Am. Chem. Soc. 1966, 88, 628–629. [Google Scholar] [CrossRef]
- Holub, J.; Vishnevskiy, Y.V.; Fanfrlík, J.; Mitzel, N.W.; Tikhonov, D.; Schwabedissen, J.; McKee, M.L.; Hnyk, D. Bromination mechanism of closo-1,2-C2B10H12 and the structure of the resulting 9-Br-closo-1,2-C2B10H11 determined by gas electron diffraction. ChemPlusChem 2020, 85, 2606–2610. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of 9,9′,12,12′-substituted cobalt bis(dicarbollide) derivatives. Russ. Chem. Bull. 2015, 64, 712–717. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Kalinin, V.N.; Lozovskaya, L.S. Formation of isomeric compounds in the halogenation of bareness and neobarenes. I. Mono- and dehalogenation of barene and neobarene. Bull. Acad. Sci. USSR Div. Chem. Sci. 1968, 17, 1683–1688. [Google Scholar] [CrossRef]
- Shernyukov, A.V.; Salnikov, G.E.; Rudakov, D.A.; Genaev, A.M. Noncatalytic bromination of icosahedral dicarboranes: The key role of anionic bromine clusters facilitating Br atom insertion into the B-H σ-bond. Inorg. Chem. 2021, 60, 3106–3116. [Google Scholar] [CrossRef]
- Palysaeva, N.V.; Gladyshkin, A.G.; Vatsadze, I.A.; Suponitsky, K.Y.; Dmitriev, D.E.; Sheremetev, A.B. N-(2-Fluoro-2,2-dinitroethyl)azoles: Novel assembly of diverse explosophoric building blocks for energetic compounds design. Org. Chem. Front. 2019, 6, 249–255. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Tsirelson, V.G.; Feil, D. Electron-density-based calculations of intermolecular energy: Case of urea. Acta Cryst. A 1999, 55, 821–827. [Google Scholar] [CrossRef]
- Dmitrienko, A.O.; Karnoukhova, V.A.; Potemkin, A.A.; Struchkova, M.I.; Kryazhevskikh, I.A.; Suponitsky, K.Y. The influence of halogen type on structural features of compounds containing α-halo-α,α-dinitroethyl moieties. Chem. Heterocycl. Comp. 2017, 53, 532–539. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Kudin, K.N., Jr.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, UK, 2004. [Google Scholar]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Bregadze, V.I. Practical synthesis of 9-methylthio-7,8-nido-carborane [9-MeS-7,8-C2B9H11]−. Some evidences of BH···X hydride-halogen bonds in 9-XCH2(Me)S-7,8-C2B9H11 (X = Cl, Br, I). J. Organomet. Chem. 2017, 849–850, 315–323. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. Synthesis of 10-methylsulfide and 10-alkylmethylsulfonium nido-carborane derivatives: B–H·π Interactions between the B–H–B hydrogen atom and alkyne group in 10-RC≡CCH2S(Me)-7,8-C2B9H11. Eur. J. Inorg. Chem. 2017, 4436–4443. [Google Scholar] [CrossRef] [Green Version]
- Suponitsky, K.Y.; Burakov, N.I.; Kanibolotsky, A.L.; Mikhailov, V.A. Multiple noncovalent bonding in halogen complexes with oxygen organics. I. Tertiary amides. J. Phys. Chem. A 2016, 120, 4179–4190. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Butterworth-Heinemann: Burlington, NJ, USA, 2009. [Google Scholar]
- Isotope Distribution Calculator and Mass Spec Plotter. Available online: https://www.sisweb.com/mstools/isotope.htm (accessed on 28 December 2021).
- APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhidkova, O.B.; Druzina, A.A.; Anufriev, S.A.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. Synthesis and Crystal Structure of 9,12-Dibromo-ortho-Carborane. Molbank 2022, 2022, M1347. https://doi.org/10.3390/M1347
Zhidkova OB, Druzina AA, Anufriev SA, Suponitsky KY, Sivaev IB, Bregadze VI. Synthesis and Crystal Structure of 9,12-Dibromo-ortho-Carborane. Molbank. 2022; 2022(1):M1347. https://doi.org/10.3390/M1347
Chicago/Turabian StyleZhidkova, Olga B., Anna A. Druzina, Sergey A. Anufriev, Kyrill Yu. Suponitsky, Igor B. Sivaev, and Vladimir I. Bregadze. 2022. "Synthesis and Crystal Structure of 9,12-Dibromo-ortho-Carborane" Molbank 2022, no. 1: M1347. https://doi.org/10.3390/M1347
APA StyleZhidkova, O. B., Druzina, A. A., Anufriev, S. A., Suponitsky, K. Y., Sivaev, I. B., & Bregadze, V. I. (2022). Synthesis and Crystal Structure of 9,12-Dibromo-ortho-Carborane. Molbank, 2022(1), M1347. https://doi.org/10.3390/M1347







