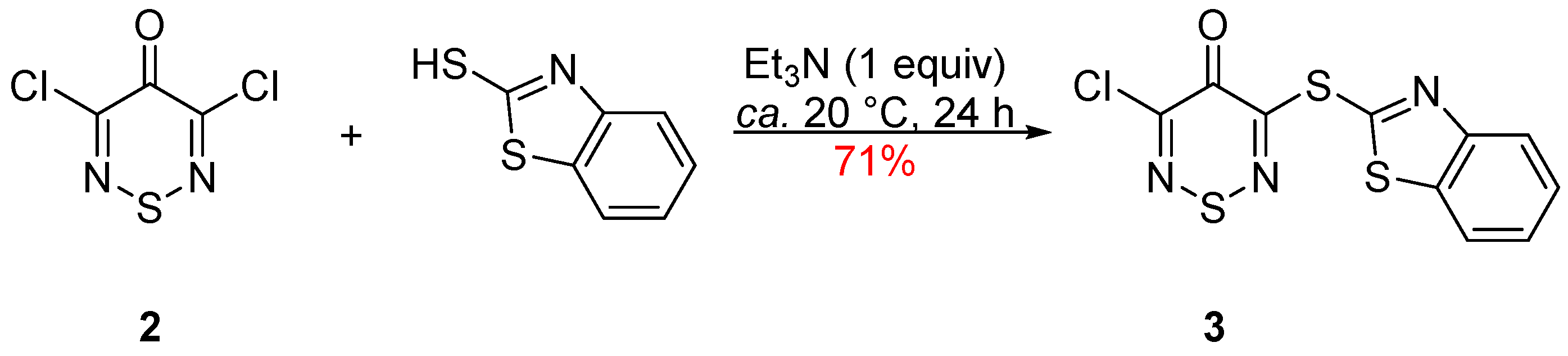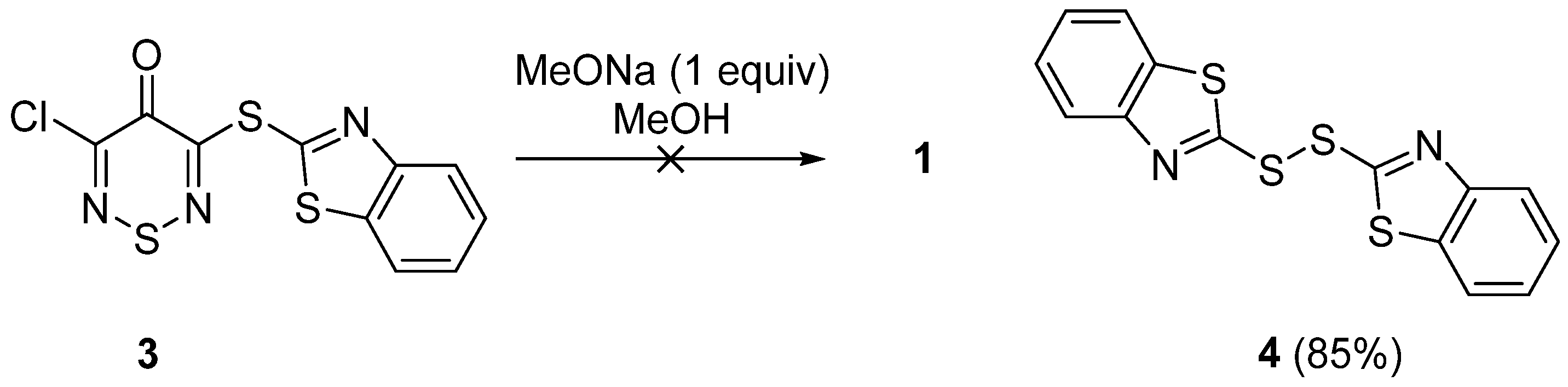2. Results and Discussion
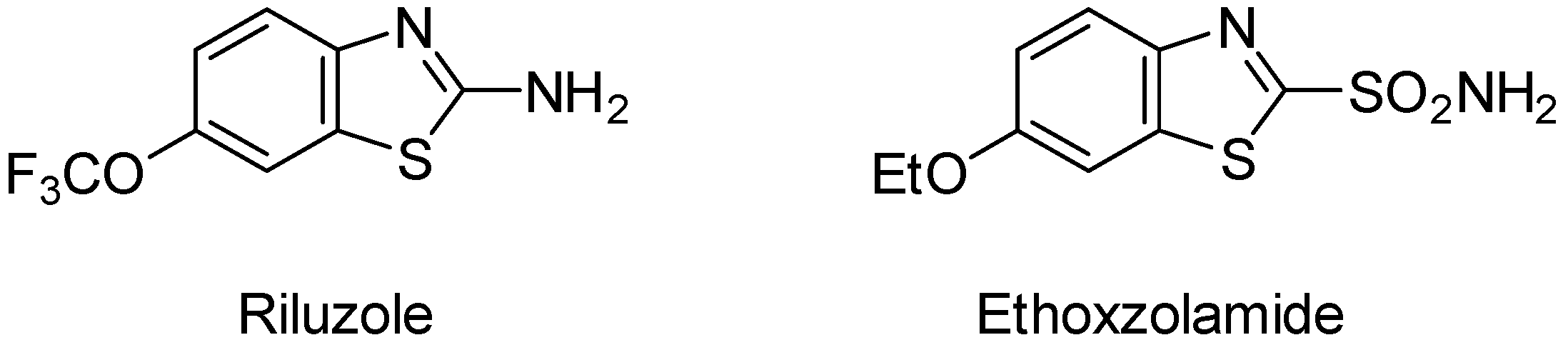
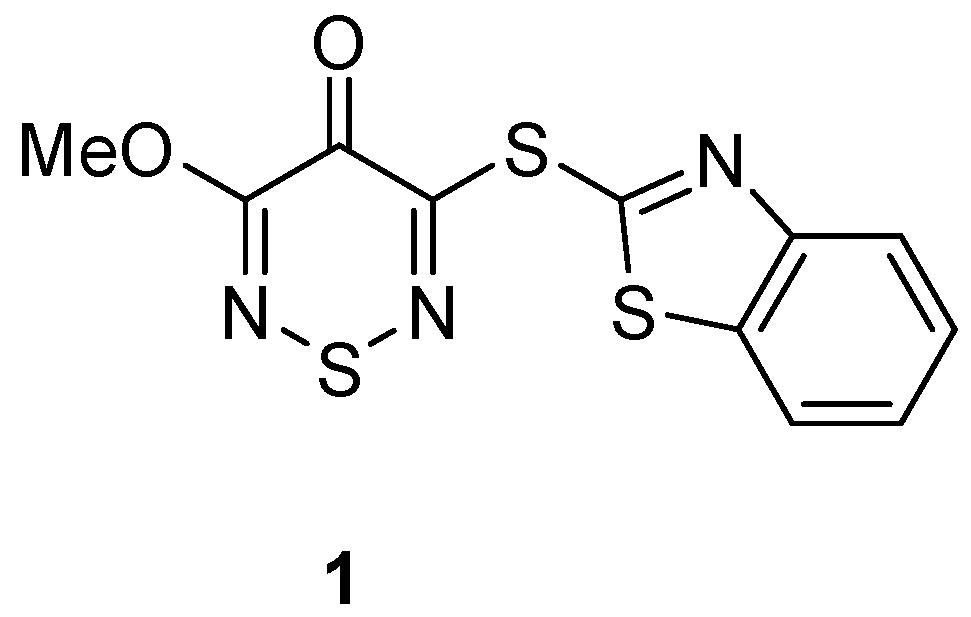
Our interest in benzothiazoles began with the study of 1,2,6-thiadiazines with activity as Large Neutral Amino Acid Transporter 1 (LAT1) inhibitors [
3]. The expression of this transporter in cells is highly upregulated in various types of human cancer that are characterized by an intense demand for amino acids for growth and proliferation, thereby making it an important drug target for cancer treatment. Our investigation required access to 3-(benzo[
d]thiazol-2-ylthio)-5-methoxy-4
H-1,2,6-thiadiazin-4-one (
1) (
Figure 2).
Early efforts to introduce the thio benzo[
d]thiazole to the known 3-chloro-5-methoxy-4
H-1,2,6-thiadiazin-4-one (conditions: benzo[
d]thiazole-2-thiol (1 equiv) and Et
3N (1 equiv), in THF, at ca. 66 °C, for 24 h) failed and only a quantitative recovery of starting thiadiazine was obtained. This was expected as the electron-releasing methoxy substituent deactivates the thiadiazine towards nucleophilic attack [
4]. As such, our attempted synthesis of 5-methoxythiadiazine
1 required first the introduction of a 2-thio benzo[
d]thiazole unit to the thiadiazine C-3 position. The reaction of 3,5-dichloro-4
H-1,2,6-thiadiazin-4-one (
2) with benzo[
d]thiazole-2-thiol (1 equiv) and Et
3N (1 equiv), in THF at ca. 20 °C, after 24 h led to the complete consumption of the starting material and, on chromatography, the isolation of benzothiazole
3 in 71% yield (
Scheme 1). Product
3 was isolated as yellow needles, mp 200–201 °C (from DCE/EtOH). FTIR spectroscopy showed aromatic
ν(C–H) stretches at 3067 and 3053 cm
−1 and a strong
ν(C=O) stretch at 1643 cm
−1, while mass spectrometry revealed a molecular ion (MH
+) peak of
m/
z 314 (100%), along with the MH
+ + 2 peak at 316 (35%), supporting the presence of one chlorine.
13C NMR spectroscopy showed the presence of four CH resonances and six quaternary carbon resonances, while a correct elemental analysis (CHN) was obtained for the molecular formula C
10H
4ClN
3OS
3, agreeing with the structure shown above (see
Supplementary Materials for the complete spectra).
Subsequently, we attempted to displace the remaining C-5 chloride by methoxide. Unfortunately, the reaction of sulfide
3 with sodium methoxide failed to produce the desired product. TLC of the reaction mixture showed only the presence of benzo[
d]thiazole-2-thiol (
Rf 0.25 (
n-hexane/DCM 20:80)), which during column chromatography oxidized to 1,2-bis(benzo[
d]thiazol-2-yl)disulfide (
4) (
Rf 0.30 (
n-hexane/DCM 40:60), mp 185–187 °C, lit. 187–189 °C [
5], isolated in 85% yield. This suggests that the 1,2,6-thiadiazine degraded during the reaction (
Scheme 2).
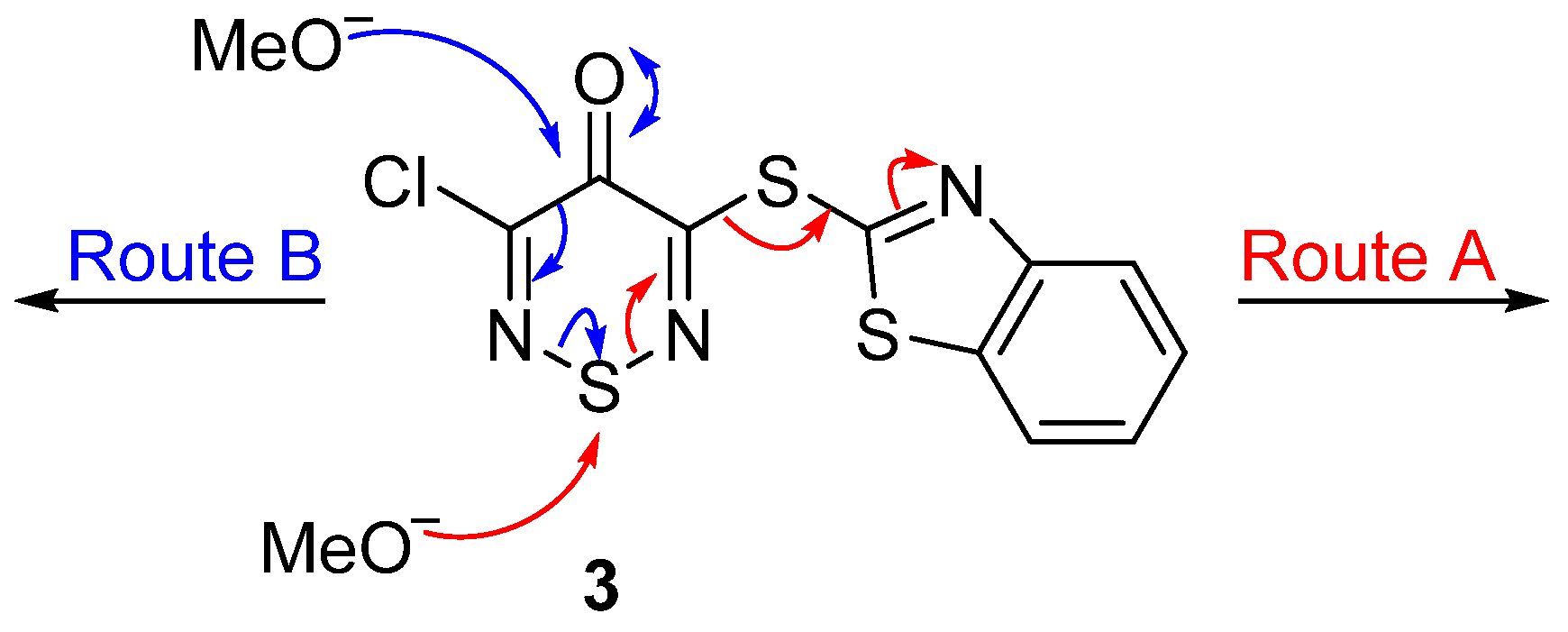
Nevertheless, the elimination of benzo[
d]thiazole-2-thiol from the thiadiazine
3 is in itself interesting. While 3-amino and 3-alkoxy-substituted 5-chlorothiadiazinones readily undergo nucleophilic substitution of the C-5 chloride with various nucleophiles [
4], the above reaction suggested that the benzothiazole sulfide was competing with the 5-chloride as a nucleofuge, but no trace of 3-chloro-5-methoxy- or 3,5-dimethoxy-1,2,6-thiadiazinones were observed (TLC) in the reaction mixture. Similarly, no trace of elemental sulfur was observed. Tentatively, methoxide attacks the ring sulfur, cleaving the thiadiazine and releasing benzo[
d]thiazole-2-thiol and a thermodynamically stable cyano group (Route A). Alternatively, nucleophilic attack at C-4 can occur, leading to ring fragmentation via cleavage of the thiadiazine C-C bond (Route B); examples of such thiadiazine ring-opening reactions are known [
6,
7]. The fate of the ring-opened species in this example remains unclear (
Scheme 3).
To further investigate the chemistry of thiadiazine sulfides, we looked at the respective methoxide substitution reaction of 3-chloro-5-(phenylthio)-4
H-1,2,6-thiadiazin-4-one (
5). Interestingly, with this sulfide the chloride substitution occurs readily to yield 3-methoxy-5-(phenylthio)-4
H-1,2,6-thiadiazin-4-one (
6) in 86% yield (
Scheme 4). This supported that the poor nucleofugality of phenyl sulfide, compared with that of benzothiazole sulfide, allowed the succesful chloride substitution of 5-phenylthiothiadiazinone
5. The ability of benzothiazole sulfide to act as a leaving group has been reported [
8,
9].
To our knowledge, compounds 3 and 6 have not been reported in the literature. We believe this new chemistry of thiadiazine sulfides to be useful in the future exploration of thiadiazinone 2 as a synthetic scaffold. The biological activity of compound 3 as a LAT1 inhibitor is under evaluation.
3. Materials and Methods
The reaction mixture was monitored by TLC using commercial glass-backed thin-layer chromatography (TLC) plates (Merck Kieselgel 60 F
254, Darmstadt, Germany). The plates were observed under UV light at 254 and 365 nm. Tetrahydrofuran (THF) was distilled over CaH
2 before use. The melting point was determined using a PolyTherm-A, Wagner & Munz, Kofler—Hotstage Microscope apparatus (Wagner & Munz, Munich, Germany). The solvent used for recrystallization is indicated after the melting point. The UV–vis spectrum was obtained using a Perkin-Elmer Lambda-25 UV–vis spectrophotometer (Perkin-Elmer, Waltham, MA, USA) and inflections are identified by the abbreviation “inf”. The IR spectrum was recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer (Shimadzu, Kyoto, Japan) with Pike Miracle Ge ATR accessory (Pike Miracle, Madison, WI, USA) and strong, medium and weak peaks are represented by s, m and w, respectively.
1H and
13C NMR spectra were recorded on a Bruker Avance 500 machine (at 500 and 125 MHz, respectively, (Bruker, Billerica, MA, USA)). Deuterated solvents were used for homonuclear lock and the signals are referenced to the deuterated solvent peaks. Attached proton test (APT) NMR studies were used for the assignment of the
13C peaks as
CH
3,
CH
2,
CH and
Cq (quaternary). The Matrix-Assisted Laser Desorption/Ionization–Time of Flight (MALDI–TOF) mass spectrum (+ve mode) was recorded on a Bruker Autoflex III Smartbeam instrument (Bruker, Billerica, MA, USA). The elemental analysis was run by the London Metropolitan University Elemental Analysis Service. 3,5-Dichloro-4
H-1,2,6-thiadiazin-4-one (
2) was prepared according to the literature procedure [
10].
3.1. 3-(Benzo[d]thiazol-2-ylthio)-5-chloro-4H-1,2,6-thiadiazin-4-one (3)
To a stirred mixture of 3,5-dichloro-4H-1,2,6-thiadiazin-4-one (2) (366 mg, 2.00 mmol) in THF (8 mL) at ca. 20 °C was added benzo[d]thiazole-2-thiol (334 mg, 2.00 mmol) followed by Et3N (279 μL, 2.00 mmol), and the mixture stirred at this temperature until complete consumption of the starting material (TLC, 24 h). The mixture was then adsorbed onto silica and chromatographed (n-hexane/DCM 20:80) to give the title compound 3 (443 mg, 71%) as yellow needles, mp 200–201 °C (from DCE/EtOH); Rf 0.46 (n-hexane/DCM 20:80); (found: C, 38.60; H, 1.39; N, 13.41. C10H4ClN3OS3 requires C, 38.28; H, 1.28; N, 13.39%); λmax(THF)/nm 270 (log ε 2.96), 310 (3.15), 344 (3.08); vmax/cm−1 3067 w and 3053 w (C–H arom), 1643 s (C=O), 1491 m, 1464 w, 1439 m, 1410 m, 1315 w, 1275 m, 1267 m, 1236 m, 1161 w, 1086 m, 1063 m, 1024 m, 1011 m, 841 m, 758 s, 739 s, 729 s, 721 s, 706 s; δH(500 MHz; DMSO-d6) 8.20 (1H, d, J 7.8, Ar CH), 8.09 (1H, d, J 7.6, Ar CH), 7.60 (1H, ddd, J 7.2, 7.2, 1.3, Ar CH), 7.56 (1H, ddd, J 7.5, 7.5, 1.2, Ar CH); δC(125 MHz; DMSO-d6) 159.1 (Cq), 158.9 (Cq), 156.6 (Cq), 151.7 (Cq), 144.4 (Cq), 136.5 (Cq), 126.8 (CH), 126.2 (CH), 122.9 (CH), 122.3 (CH); m/z (MALDI-TOF) 316 (MH+ + 2, 35%), 314 (MH+, 100), 297 (30), 166 (18).
3.2. 3-Methoxy-5-(phenylthio)-4H-1,2,6-thiadiazin-4-one (6)
To a stirred mixture of 3-chloro-5-(phenylthio)-4H-1,2,6-thiadiazin-4-one (5) (51.4 mg, 0.20 mmol) in MeOH (2 mL) at ca. 20 °C was added MeONa 1 M in MeOH (0.2 mL, 0.20 mmol), and the mixture was stirred at this temperature until complete consumption of the starting material (TLC, 1 h). The mixture was then adsorbed onto silica and chromatographed (n-hexane/DCM 50:50) to give the title compound 6 (43.3 mg, 86%) as yellow needles, mp 125–126 °C (from DCE/c-hexane); Rf 0.27 (n-hexane/DCM 50:50); (found: C, 47.73; H, 3.38; N, 10.91. C10H8N2O2S2 requires C, 47.60; H, 3.20; N, 11.10%); λmax(DCM)/nm 285 inf (log ε 4.07), 299 (4.15), 306 inf (4.13), 379 (4.01); vmax/cm−1 3038 w (C–H arom), 2959 w (C–H alip), 1626 s (C=O), 1553 w, 1520 s, 1474 m, 1466 m, 1454 m, 1443 m, 1429 w, 1342 s, 1327 m, 1307 w, 1300 w, 1246 w, 1225 m, 1184 m, 1159 w, 1070 w, 1020 w, 993 s, 957 m, 864 m, 804 s, 760 s, 721 s; δH(500 MHz; DMSO-d6) 7.51–7.54 (2H, m, Ar CH), 7.48–7.50 (3H, m, Ar CH), 3.87 (3H, s, CH3); δC(125 MHz; DMSO-d6) 160.3 (Cq), 159.0 (Cq), 152.8 (Cq), 135.2 (CH), 129.7 (CH), 129.5 (CH), 127.3 (Cq), 54.2 (CH3); m/z (MALDI-TOF) 275 (M + Na+, 18%), 253 (MH+, 100), 252 (M+, 23), 177 (17).