An Efficient Synthesis of Novel 3-[(Heteroaryl-2-ylimino)-methyl]-4-hydroxy-chromen-2-ones and Analogue of Tetrazole Derivatives and Their Antibacterial Activity
Abstract
:1. Introduction
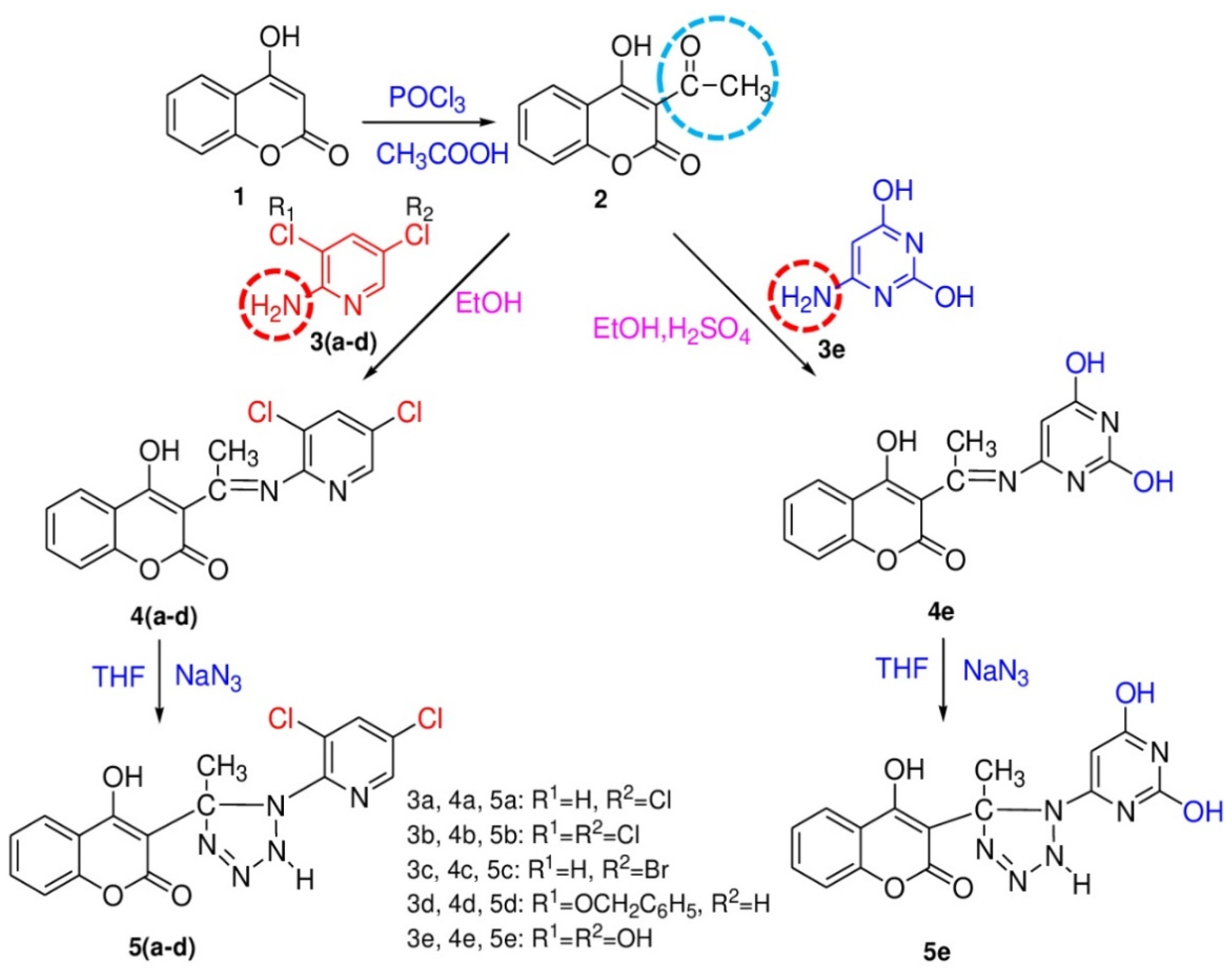
2. Results and Discussion
2.1. Chemistry
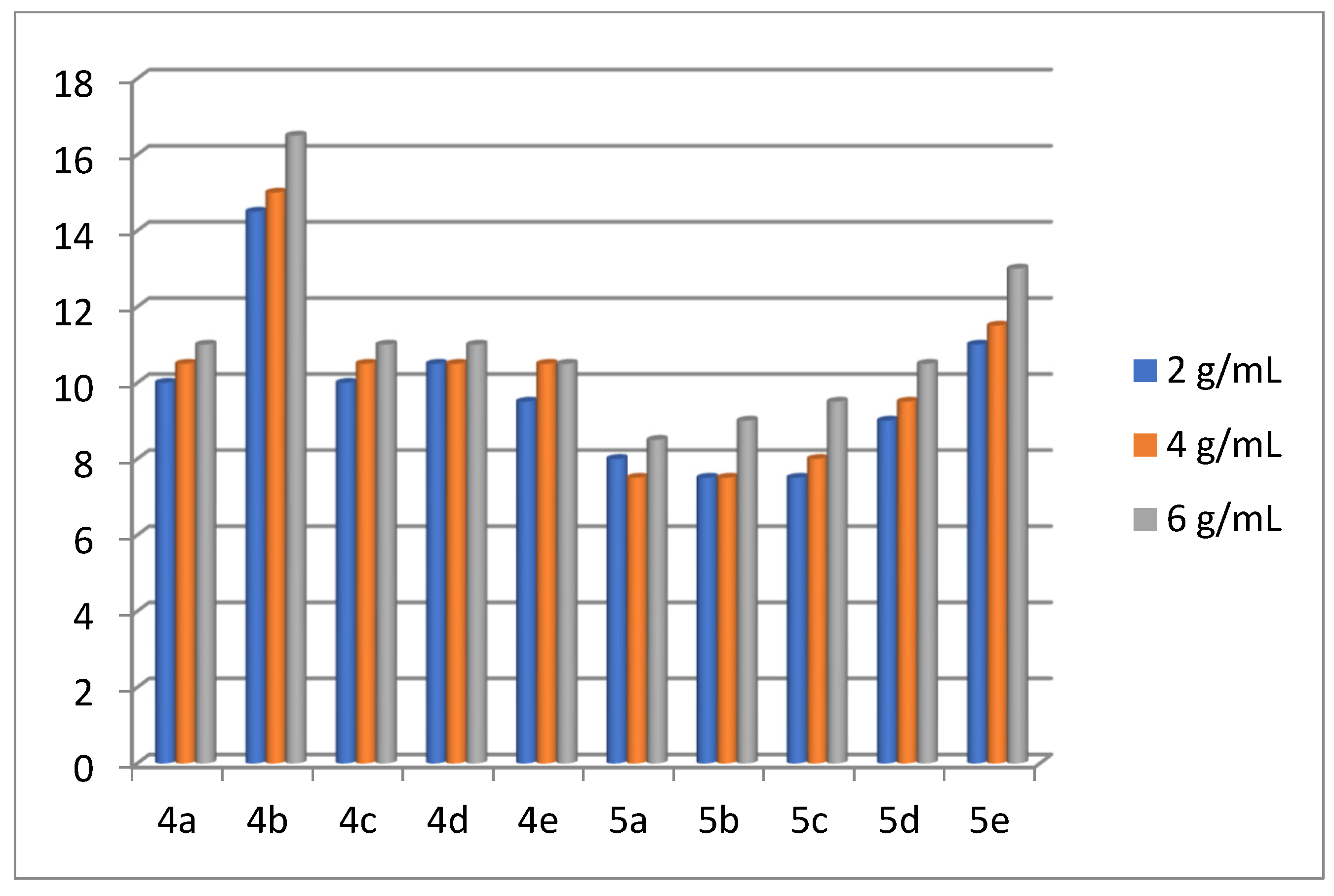
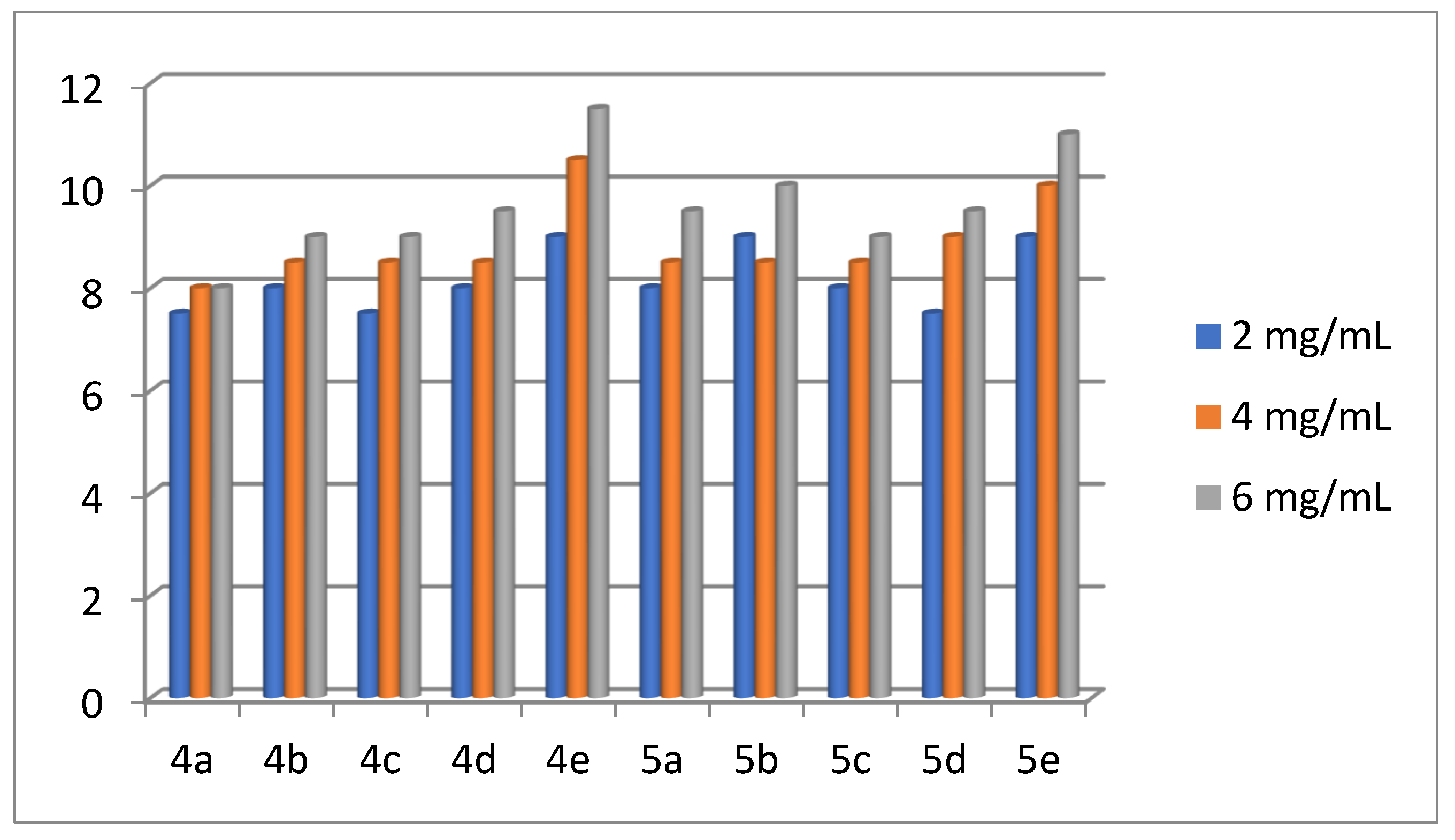
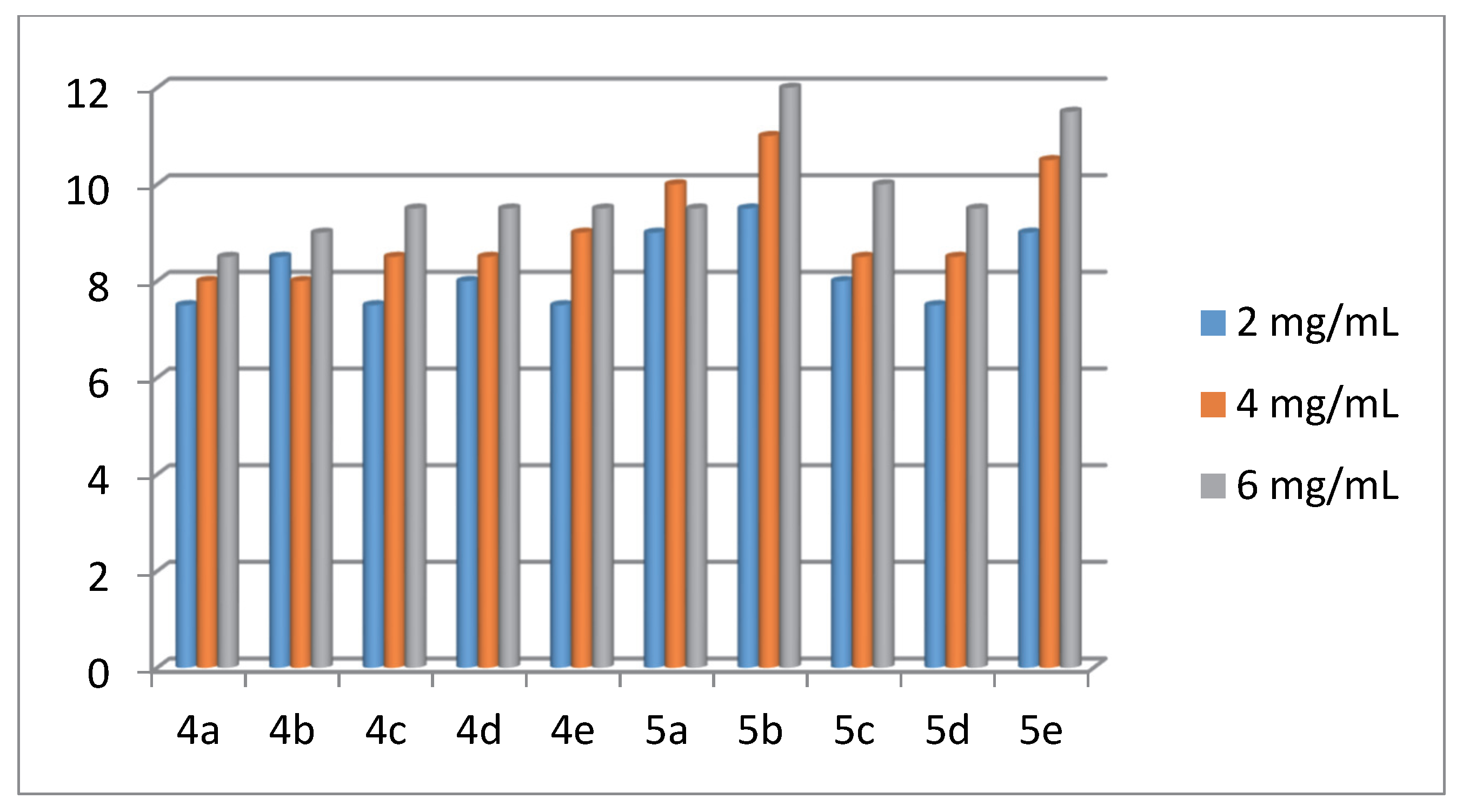
2.2. Antibacterial Activity of the Products 4(a-e) and 5(a-e)
3. Material and Methods
3.1. Materials and Basic Measurements
3.2. 3-[Pyridin-2-ylimino)-ethyl]-4-hydroxy-chromen-2-ones 4(a-e), General Procedure
3.3. 3-[1-(5-Chloro-pyridin-2-ylimino)-ethyl]-4-hydroxy-chromen-2-one
3.4. 3-[1-(3,5-Dichloro-pyridin-2-ylimino)-ethyl]-4-hydroxy-chromen-2-one
3.5. 3-[1-(5-Bromo-pyridin-2-ylimino)-ethyl]-4-hydroxy-chromen-2-one
3.6. 3-[1-(3-Benzyloxy-pyridin-2-ylimino)-ethyl]-4-hydroxy-chromen-2-one
3.7. 3-[1-(2,6-Dihydroxy-pyrimidin-4-ylimino)-ethyl]-chromen-2-one
3.8. 3-[Pyridin-2-yl)-5-methyl-2,5-dihydro-1H-tetrazol-5-yl]-4-hydroxy-chromen-2-ones 5(a-e), general procedure
3.9. 3-[1-(5-Chloro-pyridin-2-yl)-5-methyl-2,5-dihydro-1H-tetrazol-5-yl]-4-hydroxy-chromen-2-one
3.10. 3-[1-(3,5-Dichloro-pyridin-2-yl)-5-methyl-2,5-dihydro-1H-tetrazol-5-yl]-4-hydroxy-chromen-2-one
3.11. 3-[1-(5-Bromo-pyridin-2-yl)-methyl-2,5-dihydro-1H-tetrazol-5-yl]-4-hydroxy-chromen-2-one
3.12. 3-[1-(3-Benzyloxy-pyridin-2-yl)-5-methyl-2,5-dihydro-1H-tetrazol-5-yl]-4-hydroxy-chromen-2-one
3.13. 3-[1-(2,6-Dihydroxy-pyrimidin-4-yl)-5-methyl-2,5-dihydro-1H-tetrazol-5-yl]-4-hydroxy-chromen-2-one
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jameel, E.; Umar, T.; Kumar, J.; Hoda, N. Coumarin: A Privileged Scaffold for the Design and Development of Antineurodegenerative Agents. Chem. Biol. Drug Des. 2016, 87, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Cooperwood, J.; Khan, M.O. A Review of Coumarin Derivatives in Pharmacotherapy of Breast Cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [Green Version]
- Marcu, M.G.; Schulte, T.W.; Neckers, L. Novobiocin and Related Coumarins and Depletion of Heat Shock Protein 90-Dependent Signaling Proteins. JNCI J. Natl. Cancer Inst. 2000, 92, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.; Agrawal, R.; Ubaidullah, M.; Hassan, M.I.; Tarannum, N. Design, Synthesis and Validation of Anti-Microbial Coumarin Derivatives: An Efficient Green Approach. Heliyon 2019, 5, e02615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushwaha, K.; Kaushik, N.; Lata; Jain, S.C. Design and Synthesis of Novel 2H-Chromen-2-One Derivatives Bearing 1,2,3-Triazole Moiety as Lead Antimicrobials. Bioorg. Med. Chem. Lett. 2014, 24, 1795–1801. [Google Scholar] [CrossRef]
- Prusty, J.S.; Kumar, A. Coumarins: Antifungal Effectiveness and Future Therapeutic Scope. Mol. Divers. 2020, 24, 1367–1383. [Google Scholar] [CrossRef]
- Guerra, F.Q.S.; Araújo, R.S.A.; Sousa, J.P.; Silva, V.A.; Pereira, F.O.; Mendonça-Junior, F.J.B.; Barbosa-Filho, J.M.; Pereira, J.A.; Lima, E.O. A New Coumarin Derivative, 4-Acetatecoumarin, with Antifungal Activity and Association Study against Aspergillus spp. Braz. J. Microbiol. 2018, 49, 407–413. [Google Scholar] [CrossRef]
- Patel, K.; Karthikeyan, C.; Hari Narayana Moorthy, N.S.; Deora, G.S.; Solomon, V.R.; Lee, H.; Trivedi, P. Design, Synthesis and Biological Evaluation of Some Novel 3-Cinnamoyl-4-Hydroxy-2H-Chromen-2-Ones as Antimalarial Agents. Med. Chem. Res. 2012, 21, 1780–1784. [Google Scholar] [CrossRef]
- Salem, M.; Marzouk, M.; El-Kazak, A. Synthesis and Characterization of Some New Coumarins with in Vitro Antitumor and Antioxidant Activity and High Protective Effects against DNA Damage. Molecules 2016, 21, 249. [Google Scholar] [CrossRef]
- Kenchappa, R.; Bodke, Y.D.; Chandrashekar, A.; Aruna Sindhe, M.; Peethambar, S.K. Synthesis of Coumarin Derivatives Containing Pyrazole and Indenone Rings as Potent Antioxidant and Antihyperglycemic Agents. Arab. J. Chem. 2017, 10, S3895–S3906. [Google Scholar] [CrossRef] [Green Version]
- Mohareb, R.; MegallyAbdo, N. Uses of 3-(2-Bromoacetyl)-2H-Chromen-2-One in the Synthesis of Heterocyclic Compounds Incorporating Coumarin: Synthesis, Characterization and Cytotoxicity. Molecules 2015, 20, 11535–11553. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-F.; Shen, Q.-K.; Li, J.-J.; Tian, Y.-S.; Quan, Z. Synthesis and Biological Evaluation of Novel 7-Hydroxy-4-Phenylchromen-2-One–Linked to Triazole Moieties as Potent Cytotoxic Agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.; Patel, T.; Baxi, S.; Acharya, H.; Tripathi, C. Antitubercular Effect of 8-[(4-Chloro Phenyl) Sulfonyl]-7-Hydroxy-4-Methyl-2H-Chromen-2-One in Guinea Pigs. J. Pharmacol. Pharmacother. 2011, 2, 253–260. [Google Scholar] [CrossRef]
- O’Reilly, R.A.; Aggeler, P.M.; Leong, L.S. STUDIES ON THE COUMARIN ANTICOAGULANT DRUGS: THE PHARMACODYNAMICS OF WARFARIN IN MAN*. J. Clin. Invest. 1963, 42, 1542–1551. [Google Scholar] [CrossRef] [Green Version]
- Olmedo, D.; Sancho, R.; Bedoya, L.M.; López-Pérez, J.L.; del Olmo, E.; Muñoz, E.; Alcamí, J.; Gupta, M.P.; San Feliciano, A. 3-Phenylcoumarins as Inhibitors of HIV-1 Replication. Molecules 2012, 17, 9245–9257. [Google Scholar] [CrossRef]
- Bhavsar, D.; Trivedi, J.; Parekh, S.; Savant, M.; Thakrar, S.; Bavishi, A.; Radadiya, A.; Vala, H.; Lunagariya, J.; Parmar, M.; et al. Synthesis and in Vitro Anti-HIV Activity of N-1,3-Benzo[d]Thiazol-2-Yl-2-(2-Oxo-2H-Chromen-4-Yl)Acetamide Derivatives Using MTT Method. Bioorg. Med. Chem. Lett. 2011, 21, 3443–3446. [Google Scholar] [CrossRef]
- Domitrović, R.; Potočnjak, I. A Comprehensive Overview of Hepatoprotective Natural Compounds: Mechanism of Action and Clinical Perspectives. Arch. Toxicol. 2016, 90, 39–79. [Google Scholar] [CrossRef]
- Soni, R.; Durgapal, S.D.; Soman, S.S.; Georrge, J.J. Design, Synthesis and Anti-Diabetic Activity of Chromen-2-One Derivatives. Arab. J. Chem. 2019, 12, 701–708. [Google Scholar] [CrossRef]
- Servet Altay, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Prolonged Coagulopathy Related to Coumarin Rodenticide in a Young Patient: Superwarfarin Poisoning. Cardiovasc. J. Afr. 2012, 23, 9–11. [Google Scholar] [CrossRef]
- Amin, K.M.; Abou-Seri, S.M.; Awadallah, F.M.; Eissa, A.A.M.; Hassan, G.S.; Abdulla, M.M. Synthesis and Anticancer Activity of Some 8-Substituted-7-Methoxy-2H-Chromen-2-One Derivatives toward Hepatocellular Carcinoma HepG2 Cells. Eur. J. Med. Chem. 2015, 90, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Li, J.; Wu, F.-R.; Song, B.-A.; Pinaki, S.B.; Shi, L. Novel 3-(2-(3-Methyl-5-Substituted-Phenyl-4,5-Dihydropyrazol-1-Yl)-2-Oxoethoxy)-2-Substituted-Phenyl-4H-Chromen-4-One: Synthesis and Anticancer Activity. Med. Chem. 2011, 7, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, W.; Xie, Y.; Wu, J.; Liu, Y.; Liu, C.; Xu, W.; Tang, L.; Wang, J.; Zhao, G. Design, Synthesis and Biological Activity of Tetrazole-Bearing Uric Acid Transporter 1 Inhibitors. Chem. Res. Chin. Univ. 2017, 33, 49–60. [Google Scholar] [CrossRef]
- Vellalacheruvu, R.; Leela R, S.; Lk, R. Novel Route for Synthesis of Antihypertensive Activity of Tetrazole Analogues as a Carbamate and Urea Derivatives. Med. Chem. 2017, 7, 239–246. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Thampi, P.P. Synthesis and Analgesic Evaluation of Some 5-[β-(10-Phenothiazinyl)Ethyl]-1-(Acyl)-1,2,3,4-Tetrazoles. Eur. J. Med. Chem. 2004, 39, 273–279. [Google Scholar] [CrossRef]
- Lamie, P.F.; Philoppes, J.N.; Azouz, A.A.; Safwat, N.M. Novel Tetrazole and Cyanamide Derivatives as Inhibitors of Cyclooxygenase-2 Enzyme: Design, Synthesis, Anti-Inflammatory Evaluation, Ulcerogenic Liability and Docking Study. J. Enzym. Inhib. Med. Chem. 2017, 32, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Hoti, R.; Nura-Lama, A.; Mulliqi-Osmani, G.; Troni, N.; Gashi, F.; Ismaili, H.; Thaçi, V. Synthesis of 4-Triazolylamino- and 4-Benzothiazolylamino-3- Nitro-2H-[1]-Benzopyran-2-Ones and Their Antimicrobial Activity. Orbital Electron. J. Chem. 2014, 6, 184–190. [Google Scholar]
- Hoti, R.; Ismaili, H.; Vehapi, I.; Troni, N.; Mulliqi-Osmani, G.; Thaçi, V. Synthesis of new [(3-nitro-2-oxo-2H-chromen-4-ylamino)-phenyl]-phenyl-triazolidin-4-ones and their antibacterial activity. Chem. Bulg. J. Sci. Educ. 2017, 26, 262–274. [Google Scholar]
- Hoti, R.; Troni, N.; Ismaili, H.; Pllana, M.; Pacarizi, M.; Thaçi, V.; Mulliqi-Osmani, G. Synthesis of new 3-[(chromen-3-YL)-ethylideneamino]-phenyl]-thiazolidin-4-ones and their antibacterial activity. Chem. Bulg. J. Sci. Educ. 2018, 27, 109–119. [Google Scholar]
- Abdou, M.M. 3-Acetyl-4-Hydroxycoumarin: Synthesis, Reactions and Applications. Arab. J. Chem. 2017, 10, 3664–3675. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; ASM MicrobeLibrary; American Society for Microbiology: New York, NY, USA, 2009. [Google Scholar]
- Abdelmajeid, A.; Ali, a.A.; Ashour, M. Synthesis and Evaluation of in Vitro Biological Activity of New Series of Quinazolinone and Benzoxazinone Derivatives. Egypt. J. Chem. 2021, 64, 2653–2661. [Google Scholar] [CrossRef]
- Saleh, M.; Ayoub, A.; Hammady, A. Synthesis Biological Studies of Some New Heterocyclic Compound Derived from 2-Chloro-3-Formyl Quinoline and 4-(Benzyl Sulfonyl) Acetophenone. Egypt. J. Chem. 2020, 63, 4769–4776. [Google Scholar] [CrossRef]
| Nr | Molecular Formulas | Molecular Mass | Elemental Analysis % Calc/Found | mp/°C | Yield % |
|---|---|---|---|---|---|
| 4a | C16H11N2O3Cl | 314.71 | (C-61.06; H-3.52; N-8.90; O-15.25; Cl-11.26) (C-61.02; H-3.51; N-8.85; Cl: 11.28) | 204–206 | 66 |
| 4b | C16H10N2O3Cl2 | 349.15 | (C-55.03; H-2.89; N-8.02; O-13.75; Cl-20.31) (C-49.99; H-2.86; N-8.04; Cl-20.27) | 212–214 | 72 |
| 4c | C16H11N2O3Br | 359.16 | (C-53.50; H-3.09; N-7.80; O-13.36; Br-22.25) (C-53.48; H-3.12; N-7.76; Br-22.21) | 225–227 | 75 |
| 4d | C23H18N2O4 | 385.38 | (C-71.68; H-4.70; N-7.27; O-16.60) (C-71.72; H-4.67; N-7.24) | 305–307 | 38 |
| 4e | C15H11N3O5 | 313.26 | (C-57.51; H-3.54; N-13.41; O-25.54) (C-57.47; H-3.56; N-13.38) | 302–304 | 62 |
| 5a | C16H12N5O3Cl | 357.74 | (C-53.71; H-3.38; N-19.57; O-13.42; Cl-9.91) (C-53.66; H-3.42; N-19.60; Cl-9.87) | 211–213 | 65 |
| 5b | C16H11N5O3Cl2 | 392.18 | (C-48.99; H-2.83; N-17.86; O-12.24; Cl-18.08) (C-49.03; H-2.79; N-17.84; Cl-18.03) | 208–211 | 73 |
| 5c | C16H12N5O3Br | 402.19 | (C-47.78; H-3.01; N-17.41; O-11.93; Br-19.87) (C-47.82; H-2.98; N-17.38; Br-19.90) | 120–122 | 70 |
| 5d | C23H19N5O4 | 429.41 | (C-64.33; H-4.46; N-16.31; O-14.19) (C-64.29; H-4.44; N-16.28) | 224–226 | 67 |
| 5e | C15H12N6O5 | 356.29 | (C-50.56; H-3.39; N-23.59; O-22.45) (C-50.58; H-3.43; N-23.62) | 223–225 | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoti, R.; Ismaili, H.; Thaçi, V.; Mulliqi-Osmani, G.; Pllana-Zeqiri, M.; Bytyqi, A. An Efficient Synthesis of Novel 3-[(Heteroaryl-2-ylimino)-methyl]-4-hydroxy-chromen-2-ones and Analogue of Tetrazole Derivatives and Their Antibacterial Activity. Molbank 2021, 2021, M1303. https://doi.org/10.3390/M1303
Hoti R, Ismaili H, Thaçi V, Mulliqi-Osmani G, Pllana-Zeqiri M, Bytyqi A. An Efficient Synthesis of Novel 3-[(Heteroaryl-2-ylimino)-methyl]-4-hydroxy-chromen-2-ones and Analogue of Tetrazole Derivatives and Their Antibacterial Activity. Molbank. 2021; 2021(4):M1303. https://doi.org/10.3390/M1303
Chicago/Turabian StyleHoti, Ramiz, Hamit Ismaili, Veprim Thaçi, Gjyle Mulliqi-Osmani, Malësore Pllana-Zeqiri, and Agon Bytyqi. 2021. "An Efficient Synthesis of Novel 3-[(Heteroaryl-2-ylimino)-methyl]-4-hydroxy-chromen-2-ones and Analogue of Tetrazole Derivatives and Their Antibacterial Activity" Molbank 2021, no. 4: M1303. https://doi.org/10.3390/M1303
APA StyleHoti, R., Ismaili, H., Thaçi, V., Mulliqi-Osmani, G., Pllana-Zeqiri, M., & Bytyqi, A. (2021). An Efficient Synthesis of Novel 3-[(Heteroaryl-2-ylimino)-methyl]-4-hydroxy-chromen-2-ones and Analogue of Tetrazole Derivatives and Their Antibacterial Activity. Molbank, 2021(4), M1303. https://doi.org/10.3390/M1303






