Abstract
This communication describes an unprecedented substitution cascade, in which 4-methylpyridine, following deprotonation with LDA, twice acts as a carbon nucleophile in an unusual SNAr process, to form a novel triarylmethane structure. A proposed mechanism for this sequence is presented that is supported by single crystal X-ray analysis of the resulting product. We believe this unique transformation is of note as it highlights a neat and efficient entry as a single step to complex triarylmethane architectures containing both substituted phenyl and pyridyl aromatics.
1. Introduction
C-Nucleophilic aromatic substitutions are of considerable importance to synthetic chemists as they allow the creation of molecular scaffolds, in this case forming high-energy C-C bonds in few stages [1,2]. Additionally, such processes can be part of more complex reaction cascades, resulting in the rapid construction of larger architectures that arise from a well-orchestrated and typically very step-efficient process [3]. The discovery of such pathways therefore allows chemists not only to access new chemical space, but moreover enables the design of novel structures in target-oriented synthesis programs.
2. Results and Discussion
A recent synthesis program in our laboratory concerned the activation of the C–H bonds of the methyl group of 4-methylpyridine (1), owing to its acidic behaviour in the presence of extremely strong bases. Our aim was to use such in situ generated nucleophiles to add to various aromatic nitriles, to generate, following work-up, the corresponding ketones, a reaction widely reported in the literature (Figure 1) [4,5,6,7]. However, the presence and positioning of a halogen-substituent (in particular, fluoro), combined with other electron withdrawing groups on an aromatic ring can arise to create an effective alternative electrophilic site. While this method is frequently utilised to form aromatic C-heteroatom bonds [8,9,10,11], the formation of C–C without any additional form of metal catalysis remains fairly unique.

Figure 1.
Synthesis of aryl pyridylmethyl ketone via addition to nitriles [4,5,6,7].
As encountered in our work, upon deprotonation of the methylpyridine (1) starting material, the formed carbanion can competitively attack instead the aryl electron deficient centre of the 4-fluorobenzonitrile (2) acceptor (Figure 2). The instigation of ipso attack is promoted by the strong ability of the fluoro group to effectively polarise the associated carbon making it more susceptible to nucleophilic attack. Indeed, although the fluoride is a relatively good leaving group the associated C–F bond is strong, however, the desire for the system to regain aromaticity drives the fission of this bond, even at room temperature. The presence of the new group lowers the pKA of the remaining alkyl protons, as the group is electron-withdrawing, hence a second deprotonation, instigated by the original 4-methylpyridine anion acting as the base, followed by a secondary SNAr is likely more favourable than the original 4-methylpyridine anion attack on a new molecule of 4-fluorobenzonitrile (2), hence the tricyclic structure 3 is formed. No quaternary structures were observed, which provisionally indicates further nucleophilic attack is restricted due to steric hindrance.
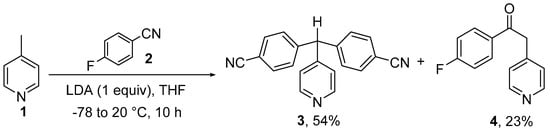
Figure 2.
The synthetic scheme by which the title compound 3 was synthesised, alongside a by-product, 4 (percentage yield calculated from the amount of 4-fluorobenzonitrile (2) starting material, with 1 treated as being in excess) [7,12].
To fully account for this initially unexpected reaction outcome, we propose the following mechanistic rationale, based on understanding of electron-deficient fluorobenzenes (Figure 3) [13]. Deprotonation of the most-acidic proton of 4-methylpyridine is expected to generate the corresponding carbanion 1a that allows a nucleophilic attack at the C–F bond of 2. This intermediate eliminates F− in order to re-gain aromaticity, forming intermediate 5. As the pKA of 5 is lower than that of 1, a base more readily deprotonates 5, forming 5a. Compound 5a enacts the comparable nucleophilic activity of 1a on another unit of 2, in order to generate the final intermediate that leads directly to 3 by eliminating F−. At this point, the reaction progresses no further as to add another unit of 2 to the central carbon would assumingly generate an intermediate that is too sterically congested or as implied by the reaction of a related system needs additional catalysis to create the tetra-substituted centre [14]. The presence of two identical benzonitrile units removes any consideration of stereoselectivity and no products containing just one benzonitrile unit were detected in any significant quantities. As noted, the initially desired chemical product, 4, [15] does indeed form albeit in low but significant yield, resulting from the addition of the carbanion 1a to the nitrile, followed by hydrolysis during work-up. We note that a related condensation has been reported to occur between 2-pyridylacetonitrile and 4-fluorobenzonitrile in DMF in the presence of potassium tert-butoxide which supports the postulated condensation procedure outlined below [16].

Figure 3.
The proposed mechanism for the formation of the title compound 3.
Considering the stoichiometry of the reagents, product distribution and the estimated/measured pKA’s of the intermediates (1 [17] 5.94, 3 and 5 [18] 5.58 ± 0.1) it was considered likely that the current reaction conditions were base limited. As such increasing the quantity of base was considered to be a useful approach to reaction optimisation. Ultimately this strategy proved beneficial with a 1.6 equivalent excess yielding the highest yield whilst limiting side reactions; a yield of 69% 3 and 24% 4. Alternatively, a larger sacrificial excess of the 4-methylpyridine anion (1a) can be used to fully consume the 4-fluorobenzonitrile (2), in our hands using a 1.5 equivalent excess of 1a gave the best results: 74% 3 and 21% 4 isolated.
3. Materials and Methods
The initial synthesis of the title compound 3 from commercially available reagents was accomplished using the following procedure. Freshly distilled diisopropylamine (1.42 mL, 10.1 mmol) under an atmosphere of nitrogen (maintained throughout the entire synthesis) was dissolved in anhydrous THF (10 mL) and the solution was cooled to −78 °C (Note commercial lithium diisopropylamide solution 2 M in THF/heptane/ethylbenzene from Aldrich (St. Louis, MO, USA), part No. 361798 can also be used although yields were slightly lower 5–8%). n-BuLi (8.4 mL, 1.2 M in hexanes, 10 mmol) was then added dropwise. After 15 min, 4-methylpyridine (0.97 mL, 10 mmol) was added dropwise, resulting in a colour change to amber. After a further 30 min, a solution of 4-fluorobenzonitrile (1.21 g, 10 mmol) in anhydrous THF (5 mL) was added dropwise. The resulting solution was stirred at −78 °C for 1 h and then allowed to warm to 20 °C. After 10 h stirring at 20 °C, the reaction mixture was removed from the nitrogen atmosphere and then concentrated in vacuo, quenched with water (20 mL) and extracted with ethyl acetate (2 × 30 mL). The combined organic phases were dried over Na2SO4, filtered and concentrated in vacuo to yield a residue which was purified by column chromatography (30% hexanes/70% EtOAc) to give solid products 3 and 4 (0.49 g (23%)). Compound 3 was further recrystallised from dichloromethane to give off white crystals (0.80 g, 54% yield).
4,4’-(Pyridin-4-ylmethylene)dibenzonitrile (3) (see Supplementary Materials) C20H13N3, Mr = 295.35 g mol−1. off white solid. 1H NMR (400 MHz, CDCl3) 8.62–8.59 (2H, m, Aryl-H), 7.65 (4H, dm, J = 8.4 Hz, Aryl-H), 7.23–7.19 (4H, dm, J = 8.4 Hz, Aryl-H), 7.02–6.98 (2H, m, Aryl-H) and 5.62 (1H, s, (Aryl)3CH). 13C NMR (101 MHz, CDCl3) 150.5 (CH), 149.7 (C), 146.0 (C), 132.7 (CH), 130.0 (CH), 124.2 (CH), 118.3 (C), 111.7 (C), 56.0 (CH). IR (neat) ν/cm−1 = 2228.6 (m, C≡N), 1604.2 (m), 1591.8 (s), 1500.0 (m), 1410.3 (s), 864.8 (m), 823.3 (s), 622.0 (s), 561.2 (s) and 537.9 (s). LC-MS: Rt = 1.73 min, m/z 296.7 [M + H+]; HR-MS calculated for C20H14N3 296.1188, found: 296.1191 (Δ = 0.3 mDa). Crystal data for CCDC 2122916: monoclinic, space group P21/n (no. 14), at T = 120 K, a = 9.2835(6), b= 17.5421(11), c = 9.6559(6) Å, β = 90.090(3)°, V= 1572.5(2) Å3, Z = 4, Dc = 1.247 g cm−3, 29095 reflections (3612 unique, Rint = 0.039), R1 = 0.044 on 2869 data with I > 2σ(I) and wR2 = 0.111 on all data (Figure 4).
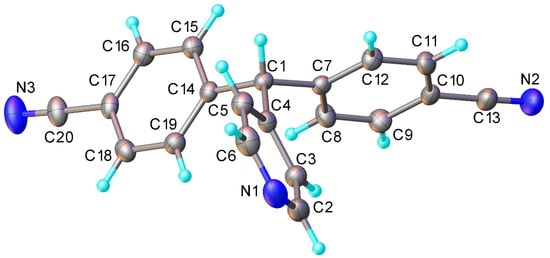
Figure 4.
The X-ray structure recorded for compound 3.
The improved synthesis condition for the title compound 3 employs the following procedure. Freshly distilled diisopropylamine (4.55 g, 45 mmol) dissolved in anhydrous THF (30 mL) under an atmosphere of nitrogen was cooled to −78 °C. To the solution was added n-BuLi (28.1 mL, 1.6 M in hexanes, 45 mmol) was then added dropwise. After 15 min, 4-methylpyridine (4.19 g, 45 mmol) in anhydrous THF (20 mL) was slowly (5 min) added. After complete addition the mixture was stirred for 15 min and a solution of 4-fluorobenzonitrile (3.63 g, 30 mmol) in anhydrous THF (20 mL) was added over 10 min. The resulting solution was stirred at −78 °C for 1 h and then allowed to warm to 20–25 °C (RT). After 10 h stirring at RT, the reaction mixture was concentrated in vacuo, quenched with water (20 mL) and extracted with ethyl acetate (3 × 50 mL). The combined organic phases were dried over Na2SO4, filtered and concentrated in vacuo to yield a residue which was purified by column chromatography (30% hexanes/70% EtOAc) to give solid products 3 (3.27 g 74%) and 4 (1.36 g 21%). Compound 3 was recrystallised from dichloromethane to give cream crystals (2.88 g, 65% yield).
4. Conclusions
In conclusion, we have accomplished an efficient synthesis of a complex tricyclic system 3 by an SNAr reaction. A mechanistic rationale accounting for this transformation is proposed. Due to the novelty of both this compound and the simplicity of the method, we believe such intriguing entities hold interest as they represent convenient routes to new triarylmethane compounds.
Supplementary Materials
The following are available online. NMR, IR and mass spectra as well as XRD data for the compounds 3 and 4.
Author Contributions
The practical investigation and data analysis was accomplished by B.M.J.L. and A.J.N. The preparation of the original draft was performed by A.J.N. The funding acquisition, supervision as well as reviewing and editing the draft was completed by I.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge financial support from Thomas Swan and Co. Ltd. during his PhD studies (A.J.N., RF030570).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Supporting data available (link to supporting data file submitted).
Acknowledgments
We furthermore are grateful to Andrei Batsanov (Durham University) for solving the X-ray crystal structure of compounds 3 and 4.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the work, nor the decision to publish the results.
References
- Bunnett, J.F.; Zahler, R.E. Aromatic Nucleophilic Substitution Reactions. Chem. Rev. 1951, 49, 273–412. [Google Scholar] [CrossRef]
- Rohrbach, S.; Smith, A.J.; Pang, J.H.; Poole, D.L.; Tuttle, T.; Chiba, S.; Murphy, J.A. Concerted Nucleophilic Aromatic Substitution Reactions. Angew. Chem. 2019, 58, 16368–16388. [Google Scholar] [CrossRef] [PubMed]
- Bella, M.; Kobbelgaard, S.; Jørgensen, K.A. Organocatalytic regio- and asymmetric C-selective S(N)Ar reactions-stereoselective synthesis of optically active spiro-pyrrolidone-3,3′-oxoindoles. J. Am. Chem. Soc. 2005, 127, 3670–3671. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Yokoyama, S.; Asahara, H.; Nishiwaki, N. Three Step Synthesis of Fully and Differently Arylated Pyridines. Eur. J. Org. Chem. 2020, 31, 466–474. [Google Scholar] [CrossRef]
- Stefanidis, D.; Bunting, J.W. Rate-equilibrium relationships for the deprotonation of 4-phenacylpyridines and 4-phenacylpyridinium cations. J. Am. Chem. 1990, 112, 3163–3168. [Google Scholar] [CrossRef]
- Chen, H.Y.; Kim, S.; Wu, J.Y.; Birzin, E.T.; Chan, W.; Yang, Y.T.; Dahllund, J.; DiNinno, F.; Rohrer, S.P.; Schaeffer, J.M.; et al. Estrogen receptor ligands. Part 3: The SAR of dihydrobenzoxathiin SERMs. Bioorg. Med. Chem. Lett. 2004, 14, 2551–2554. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I.R. Continuous-Flow Synthesis of 2H-Azirines and Their Diastereoselective Transformation to Aziridines. Synlett 2016, 27, 159–163. [Google Scholar] [CrossRef][Green Version]
- Utsugi, Y.; Kobuchi, H.; Kawamura, Y.; Atito, A.S.A.; Nagao, M.; Isoda, H.; Miyamae, Y. Importance of the proximity and orientation of ligand-linkage to the design of cinnamate-GW9662 hybrid compounds as covalent PPAR agonists. Molecules 2019, 24, 2019. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Chae, B.; Seo, H.; Jung, Y.M.; Lee, S.W. Structural characterization of triphenylamine (TPA)-based polymers during the oxidative reaction by two-dimensional (2D) infrared correlation study. J. Mol. Struct. 2014, 1069, 200–204. [Google Scholar] [CrossRef]
- Wilshire, J.J.F.K.J.; Trantino, G.G.J.G.; Mackay, M.; Trantino, G.G.J.G.; Wilshire, J.J.F.K.J. The Reaction of Some N-(Nitrophenyl)azoles With Alkali: Preparation of the Corresponding Azoxybenzenes. X-Ray Structure of 2, 2′-Bis(1″,2″,4″-triazol-1″-yl)azoxybenzene. Aust. J. Chem. 1993, 46, 417–425. [Google Scholar]
- Nicholls, A.J.; Baxendale, I.R. Benzo[1,2,3]dithiazole Compounds: A History of Synthesis and Their Renewed Applicability in Materials and Synthetic Chemistry, Originating from the Herz Reaction. Reactions 2021, 2, 175–208. [Google Scholar] [CrossRef]
- Mente, S.; Arnold, E.; Butler, T.; Chakrapani, S.; Chandrasekaran, R.; Cherry, K.; Dirico, K.; Doran, A.; Fisher, K.; Galatsis, P.; et al. Ligand-protein interactions of selective casein kinase 1δ inhibitors. J. Med. Chem. 2013, 56, 6819–6828. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, J.M.; McPake, C.B.; Murray, C.B.; Sandford, G. Synthesis of polyfluorinated terphenyl and styrene derivatives by palladium catalysed C-F bond activation of polyfluoronitroaromatic substrates. J. Fluor. Chem. 2016, 181, 51–55. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Kim, B.S.; Wu, C.; Mao, J.; Walsh, P.J. Palladium-catalysed synthesis of triaryl(heteroaryl)methanes. Nat. Commun. 2017, 8, 14641. [Google Scholar] [CrossRef] [PubMed]
- Seerden, J.-P.G.; Leusink-Ionescu, G.; Leguijt, R.; Saccavini, C.; Gelensa, E.; Dros, B.; Woudenberg-Vrenken, T.; Molema, G.; Kamps, J.A.A.M.; Kellogg, R.M. Syntheses and structure–activity relationships for some triazolyl p38α MAPK inhibitors. Bioorganic Med. Chem. Lett. 2014, 24, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, S.M.; Rashidi, M. Acidity of osmium tetroxide (OsO4) towards coordination with pyridine and its derivatives. Polyhedron 2007, 26, 1476–1482. [Google Scholar] [CrossRef]
- Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02. Available online: https://www.acdlabs.com/products/percepta/predictors/pka/index.php (accessed on 19 November 2021).
- Sterckx, H.; Houwer, J.D.; Mensch, C.; Herrebout, W.; Tehrani, K.A.; Maes, B.U.W. Base metal-catalyzed benzylic oxidation of (aryl)(heteroaryl)methanes with molecular oxygen. Beilstein J. Org. Chem. 2016, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).